Dr. Angela Messina publications
18 publications on ISI journals
1) Mono-allelic p.R37H Dehydrodolichyl Diphosphate Synthase variants lead to protein glycosylation defects, aberrant lipid profiles and interneuron scarcity in a novel mouse model of progressive epileptic encephalopathy
A.Da Silva, S.B.Tene Tadoum, I.J.J.Muffels, R.Budhraja, L.Sturiale, A.Messina, M.Giladi, M.Taherzadeh, M.Fazeli, E.Bonneil, S.Khan, D.te Vruchte, Y.Yamanaka, G.Di Cristo, F.F.Hamdan, F.M.Platt, S.Tomatsu, Y.Haitin, T.Kozicz, P.Thibault, D.Garozzo, A.Pandey, E.Morava, E.Rossignol, A.V.Pshezhetsky
bioRxiv
- 2025
DOI:
https://doi.org/10.1101/2025.08.15.670547
Developmental delay and seizures with or without movement abnormalities (OMIM 617836) caused by heterozygous pathogenic variants in the
DHDDS gene (DHDDS-CDG) is a rare genetic disease that belongs to the progressive encephalopathy spectrum. It results in developmental delay in affected children, accompanied by myoclonus, seizures, ataxia and tremor, which worsens over time.
DHDDS encodes a subunit of a DHDDS/NUS1 cis-prenyltransferase (
cis-PTase), a branch point enzyme of the mevalonate pathway essential for N-linked glycosylation. We describe the first mouse model of this disease,
DhddsR37H+/- strain, heterozygous for the human recurrent de novo c.110G>A:p.R37H pathogenic variant.
DhddsR37H+/- mice present with seizures, myoclonus and memory deficits associated with reduced density or/and maturity of inhibitory interneurons in the cortex. Multiomics analyses of mouse CNS tissues, together with the enzymatic/structural characterization of the R37H DHDDS mutant protein, reveal that the variant produces a catalytically inactive enzyme and results in a brain dolichol deficit, aberrant glycosylation of brain glycoproteins, including those involved in synaptic transmission, and major perturbations in the CNS proteome and lipidome. Acetazolamide, a carbonic anhydrase inhibitor clinically approved for treatment of glaucoma, epilepsy, and intracranial hypertension, and successfully used "off-label" to treat genetic movement disorders, drastically reduces seizure susceptibility to pentylenetetrazol in
DhddsR37H+/- mice, suggesting potential therapeutic value of using this drug in human DHDDS-CDG patients. Together, our results define
cis-PTase as a master regulator of CNS development and function and establish that its monoallelic debilitating variants cause a novel Congenital Disorder of Glycosylation, associated with aberrant levels of neuronal proteins and lipids.

2) MAN2A2-related glycosylation defects in autism and cognitive delay
S.Treccarichi, M.Vinci, L.Cirnigliaro, A.Messina, A.Palmigiano, F.Pettinato, A.Musumeci, V.Chiavetta, S.Saccone, L.Sturiale, F.Calì, R.Barone
Scientific Reports
15,
24471
- 2025
DOI:
https://doi.org/10.1038/s41598-025-09400-5
Glycosylation is a post-translational modification essential for proper protein folding and function, with significant roles in diverse biological processes, including neurogenesis. MAN2A2 enzyme is required for proper N-glycan trimming/maturation in the N-glycosylation pathway. Whole-exome sequencing of a trio revealed two potentially causative variants in the MAN2A2 gene in a patient with autism spectrum disorder (ASD) and cognitive delay. The first variant, c.1679G > A (p.Arg560Gln), was inherited from the unaffected father. It is located within the alpha-mannosidase middle functional domain, a region essential for mannose metabolism and alpha-mannosidase enzymatic activity. The second variant, c.3292C > T (p.Gln1098Ter), was inherited from the mother and it generated a premature stop codon. These variants resulted in a compound heterozygous condition in the patient. Prediction using the DOMINO tool suggested an autosomal recessive inheritance pattern. Notably, the MAN2A2 gene is highly expressed in several brain regions. The encoded enzyme, an alpha-mannosidase, is localized to the Golgi apparatus, the cellular organelle where the processing and maturation of N-glycans occurs. In silico analyses consistently classified both variants as likely pathogenic, supported by structural prediction analyses that indicated significant disruptions in protein architecture. Glycosylation analyses demonstrated impaired N-glycosylation, evidenced by the accumulation of immature serum glycoprotein N-glycans including disease-specific hybrid-type species. Further investigations are essential to elucidate the role of this gene in ASD and cognitive delay.
3) Severe kidney dysfunction in sialidosis mice reveals an essential role for neuraminidase 1 in reabsorption
I.Kho, E.P.Demina, X.Pan, I.Londono, C.W.Cairo, L.Sturiale, A.Palmigiano, A.Messina, D.Garozzo, R.Ung, F.Mac-Way, E.Bonneil, P.Thibault, M.Lemaire, C.R.Morales, A.V.Pshezhetsky
JCI Insight
8(20),
166470
- 2023
DOI:
https://doi.org/10.1172/jci.insight.166470
Sialidosis is an ultra-rare multisystemic lysosomal disease caused by mutations in the neuraminidase 1 (NEU1) gene. The severe type II form of the disease manifests with a prenatal/infantile or juvenile onset, bone abnormalities, severe neuropathology, and visceromegaly. A subset of these patients present with nephrosialidosis, characterized by abrupt onset of fulminant glomerular nephropathy. We studied the pathophysiological mechanism of the disease in 2 NEU1-deficient mouse models, a constitutive Neu1-knockout, Neu1ΔEx3, and a conditional phagocyte-specific knockout, Neu1Cx3cr1ΔEx3. Mice of both strains exhibited terminal urinary retention and severe kidney damage with elevated urinary albumin levels, loss of nephrons, renal fibrosis, presence of storage vacuoles, and dysmorphic mitochondria in the intraglomerular and tubular cells. Glycoprotein sialylation in glomeruli, proximal distal tubules, and distal tubules was drastically increased, including that of an endocytic reabsorption receptor megalin. The pool of megalin bearing O-linked glycans with terminal galactose residues, essential for protein targeting and activity, was reduced to below detection levels. Megalin levels were severely reduced, and the protein was directed to lysosomes instead of the apical membrane. Together, our results demonstrated that desialylation by NEU1 plays a crucial role in processing and cellular trafficking of megalin and that NEU1 deficiency in sialidosis impairs megalin-mediated protein reabsorption.
4) N-acetylneuraminate pyruvate lyase controls sialylation of muscle glycoproteins essential for muscle regeneration and function
A.Da Silva, J.Dort, Z.Orfi, X.Pan, S.Huang, I.Kho, E.Heckel, G.Muscarnera, P.Piet van Vliet, L.Sturiale, A.Messina, D.Romeo, C.D.M.van Karnebeek, X.Wen, A.Hinek, T.Molina, G.Andelfinger, B.Ellezam, Y.Yamanaka, H.J.Olivos, C.R.Morales, J.Joyal, D.J.Lefeber, D.Garozzo, N.A.Dumont, A.V.Pshezhetsky
Science Advances
9(26)
- 2023
DOI:
https://doi.org/10.1126/sciadv.ade6308
Deleterious variants in N-acetylneuraminate pyruvate lyase (NPL) cause skeletal myopathy and cardiac edema in humans and zebrafish, but its physiological role remains unknown. We report generation of mouse models of the disease: NplR63C, carrying the human p.Arg63Cys variant, and Npldel116 with a 116-bp exonic deletion. In both strains, NPL deficiency causes drastic increase in free sialic acid levels, reduction of skeletal muscle force and endurance, slower healing and smaller size of newly formed myofibers after cardiotoxin-induced muscle injury, increased glycolysis, partially impaired mitochondrial function, and aberrant sialylation of dystroglycan and mitochondrial LRP130 protein. NPL-catalyzed degradation of sialic acid in the muscle increases after fasting and injury and in human patient and mouse models with genetic muscle dystrophy, demonstrating that NPL is essential for muscle function and regeneration and serves as a general marker of muscle damage. Oral administration of N-acetylmannosamine rescues skeletal myopathy, as well as mitochondrial and structural abnormalities in NplR63C mice, suggesting a potential treatment for human patients.
5) HILIC-UPLC-MS for high throughput and isomeric N-glycan separation and characterization in Congenital Disorders Glycosylation and human diseases
A.Messina, A.Palmigiano, F.Esposito, A.Fiumara, A.Bordugo, R.Barone, L.Sturiale, J.Jaeken, D.Garozzo
Glycoconjugate Journal
38(2),
201-211
- 2021
DOI:
https://doi.org/10.1007/s10719-020-09947-7
N-glycan analyses may serve uncovering disease-associated biomarkers, as well as for profiling distinctive changes supporting diagnosis of genetic disorders of glycan biosynthesis named congenital disorders of glycosylation (CDG). Strategies based on liquid chromatography (LC) preferentially coupled to electrospray ionization (ESI) - mass spectrometry (MS) have emerged as powerful analytical methods for N-glycan identification and characterization. To enhance detection sensitivity, glycans are commonly labelled with a functional tag prior to LC-MS analysis. Since most derivatization techniques are notoriously time-consuming, some commercial analytical kits have been developed to speed up N-deglycosylation and N-glycan labelling of glycoproteins of pharmaceutical and biological interest such as monoclonal antibodies (mAbs). We exploited the analytical capabilities of RapiFluor-MS (RFMS) to perform, by a slightly modified protocol, a detailed N-glycan characterization of total serum and single serum glycoproteins from specific patients with CDG (MAN1B1-CDG, ALG12-CDG, MOGS-CDG, TMEM199-CDG). This strategy, accomplished by Hydrophilic Interaction Chromatography (HILIC)-UPLC-ESI-MS separation of the RFMS derivatized N-glycans, allowed us to uncover structural details of patients serum released N-glycans, thus extending the current knowledge on glycan profiles in these individual glycosylation diseases. The applied methodology enabled to differentiate in some cases either structural isomers and isomers differing in the linkage type. All the here reported applications demonstrated that RFMS method, coupled to HILIC-UPLC-ESI-MS, represents a sensitive high throughput approach for serum N-glycome analysis and a valuable option for glycan detection and separation particularly for isomeric species.
6) N-Glycomics of Human Erythrocytes
R.O.Bua, A.Messina, L.Sturiale, R.Barone, D.Garozzo, A.Palmigiano
International Journal of Molecular Sciences
22(15),
8063
- 2021
DOI:
https://doi.org/10.3390/ijms22158063
Glycosylation is a complex post-translational modification that conveys functional diversity to glycoconjugates. Cell surface glycosylation mediates several biological activities such as induction of the intracellular signaling pathway and pathogen recognition. Red blood cell (RBC) membrane N-glycans determine blood type and influence cell lifespan. Although several proteomic studies have been carried out, the glycosylation of RBC membrane proteins has not been systematically investigated. This work aims at exploring the human RBC N-glycome by high-sensitivity MALDI-MS techniques to outline a fingerprint of RBC N-glycans. To this purpose, the MALDI-TOF spectra of healthy subjects harboring different blood groups were acquired. Results showed the predominant occurrence of neutral and sialylated complex N-glycans with bisected N-acetylglucosamine and core- and/or antennary fucosylation. In the higher mass region, these species presented with multiple N-acetyllactosamine repeating units. Amongst the detected glycoforms, the presence of glycans bearing ABO(H) antigens allowed us to define a distinctive spectrum for each blood group. For the first time, advanced glycomic techniques have been applied to a comprehensive exploration of human RBC N-glycosylation, providing a new tool for the early detection of distinct glycome changes associated with disease conditions as well as for understanding the molecular recognition of pathogens.
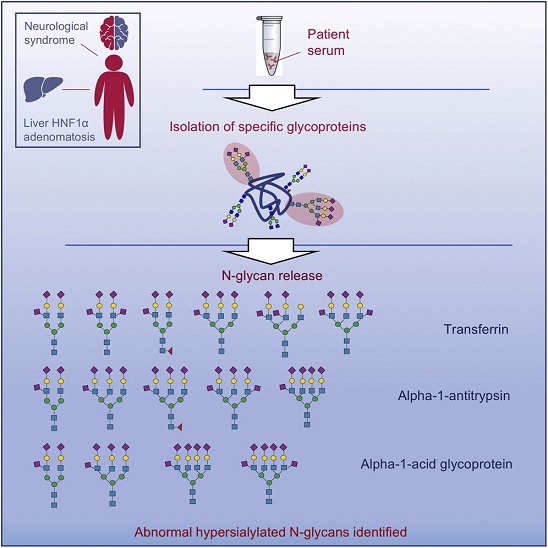
7) Aberrant sialylation in a patient with a HNF1α variant and liver adenomatosis
L.Sturiale, M.C.Nassogne, A.Palmigiano, A.Messina, I.Speciale, R.Artuso, G.Bertino, N.Revencu, X.Stephénne, C.De Castro, G.Matthijs, R.Barone, J.Jaeken, D.Garozzo
iScience
24(4),
102323
- 2021
DOI:
https://doi.org/10.1016/j.isci.2021.102323
Glycosylation is a fundamental post-translational modification of proteins that boosts their structural diversity providing subtle and specialized biological properties and functions. All those genetic diseases due to a defective glycan biosynthesis and attachment to the nascent glycoproteins fall within the wide area of congenital disorders of glycosylation (CDG), mostly causing multisystem involvement. In the present paper, we detailed the unique serum N-glycosylation of a CDG-candidate patient with an unexplained neurological phenotype and liver adenomatosis harboring a recurrent pathogenic
HNF1α variant. Serum transferrin isoelectric focusing showed a surprising N-glycosylation pattern consisting on hyposialylation, as well as remarkable hypersialylation. Mass spectrometry-based glycomic analyses of individual serum glycoproteins enabled to unveil hypersialylated complex N-glycans comprising up to two sialic acids per antenna. Further advanced MS analysis showed the additional sialic acid is bonded through an α2-6 linkage to the peripheral N-acetylglucosamine residue.
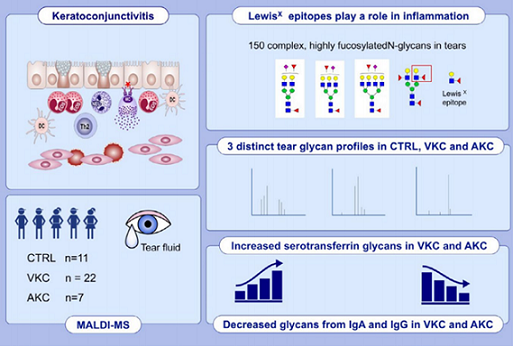
8) Tear N-glycomics in vernal and atopic keratoconjunctivitis
A.Messina, A.Palmigiano, C.Tosto, D.Romeo, L.Sturiale, D.Garozzo, A.Leonardi
Allergy
76(8),
2500-2509
- 2021
DOI:
https://doi.org/10.1111/all.14775
Purpose: Tear fluid
N-Glycome from patients affected with vernal (VKC) and atopic keratoconjunctivitis (AKC) was investigated to identify specific changes in tears and to recognize possible glyco-biomarkers.
Methods: The analysis of the
N-glycans was performed using matrix-assisted laser desorption ionization mass spectrometry on single tear samples. Tears from control normal subjects (CTRL), VKC and AKC patients were processed and treated with peptide
N-glycosidase F (PNGase F) to deglycosylate
N-glycoproteins. Released
N-glycans were purified, permethylated, and analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry and tandem mass spectrometry (MALDI-TOF MS and MALDI-TOF MS/MS).
Results: More than 150 complex
N-glycans, including highly fucosylated biantennary, triantennary, tetra-antennary, and bisecting species, were observed in our spectra. Three distinct patterns for CTRL, VKC, and AKC patients were identified in terms of relative intensities for some
N-glycans structures. Major variations involved bisecting and hyperfucosylated glycoforms.
The most intense ions were associated with species at
m/z 1907.0 (asialo, agalacto, bisected, biantennary structure-NGA2B) in CTRL MS profiles, at
m/z 2605.3 and 2966.5 in VKC, and at
m/z 2792.4 in AKC corresponding to a well-known biantennary, disialylated
N-glycan. Several peaks were associated with structures bearing one or two Lewis X epitopes. Structures were confirmed by MS/MS analysis. Quantitative differences among the three groups were statistically significant.
Conclusions: Tear MS profiles are rich in specific glycoforms, particularly those with a high fucosylation degree, indicating both core and peripheral decoration. Tear
N-glycome analysis provided important information for a better comprehension of VKC and AKC alterations at the molecular level.
9) ALG12-CDG: novel glycophenotype insights endorse the molecular defect
L.Sturiale, S.Bianca, D.Garozzo, A.Terracciano, E.Agolini, A.Messina, A.Palmigiano, F.Esposito, Ch.Barone, A.Novelli, A.Fiumara, J.Jaeken, R.Barone
Glycoconjugate journal
36,
461-472
- 2019
DOI:
https://doi.org/10.1007/s10719-019-09890-2
Congenital disorders of glycosylation (CDG) are genetic diseases characterized by deficient synthesis (CDG type I) and/or abnormal processing (CDG type II) of glycan moieties linked to protein and lipids. The impact of the molecular defects on protein glycosylation and in turn on the clinical phenotypes of patients with CDG is not yet understood. ALG12-CDG is due to deficiency of ALG12 α1,6-mannosyltransferase that adds the eighth mannose residue on the dolichol-PP-oligosaccharide precursor in the endoplasmic reticulum. ALG12-CDG is a severe multisystem disease associated with low to deficient serum immunoglobulins and recurrent infections. We thoroughly investigated the glycophenotype in a patient with novel ALG12 variants and immunodeficiency. We analyzed serum native transferrin, as first line test for CDG and we profiled serum IgG and total serum N-glycans by a combination of consolidated (N-glycan analysis by MALDI MS) and innovative mass spectrometry-based protocols, such as GlycoWorks RapiFluor N-glycan analysis coupled with LC-ESI MS. Intact serum transferrin showed, as expected for a CDG type I defect, underoccupancy of N-glycosylation sites. Surprisingly, total serum proteins and IgG N-glycans showed some specific changes, consisting in accumulating amounts of definite high-mannose and hybrid structures. As a whole, ALG12-CDG behaves as a dual CDG (CDG-I and II defects) and it is associated with distinct, abnormal glycosylation of total serum and IgG N-glycans. Glycan profiling of target glycoproteins may endorse the molecular defect unraveling the complex clinical phenotype of CDG patients.
10) CSF N-glycoproteomics for early diagnosis in Alzheimer’s disease
A.Palmigiano, R.Barone, L.Sturiale, C.Sanfilippo, R.O.Bua, D.Romeo, A.Messina, M.L.Capuana, T.Maci, F.Le Pira, M.Zappia, D.Garozzo
Journal of Proteomics
131,
29-37
- 2016
DOI:
https://doi.org/10.1016/j.jprot.2015.10.006
This work aims at exploring the human CSF (Cerebrospinal fluid) N-glycome by MALDI MS techniques, in order to assess specific glycosylation pattern(s) in patients with Alzheimer’s disease (n:24) and in subjects with mild cognitive impairment (MCI) (n:11), these last as potential AD patients at a pre-dementia stage. For comparison, 21 healthy controls were studied. We identified a group of AD and MCI subjects (about 40-50% of the studied sample) showing significant alteration of CSF N-glycome profiling, consisting of a decrease in the overall sialylation degree and an increase in species bearing bisecting GlcNAc. Noteworthy, all the MCI patients that converted to AD within the clinical follow-up, had an abnormal CSF glycosylation profile. Based on the studied cohort, CSF glycosylation changes may occur before an AD clinical onset. Previous studies specifically focused on the key role of glycosyltransferase GnT-III on AD-pathogenesis, addressing the patho-mechanism to specific sugar modification of BACE-1 glycoprotein with bisecting GlcNAc. Our patients addressed protein N-glycosylation changes at an early phase of the whole biomolecular misregulation on AD, pointing to CSF N-glycome analyses as promising tool to enhance early detection of AD and also suggesting alternative therapeutics target molecules, such as specific glyco-enzymes.
11) TEAR FLUID N-GLYCAN PROFILING TO INVESTIGATE BIOMARKERS IN VERNAL AND ATOPIC KERATOCONJUNCTIVITIS
A.Leonardi, C.Tosto, A.Messina, A.Palmigiano, R.O.Bua, D.Romeo, L.Sturiale, D.Garozzo
Investigative Ophthalmology & Visual Science
56(7),
5875
- 2015
Proteomics and glycogene-chips studies revealed that several tear proteins are glycosylated. Glycoproteins play a role in different function of the ocular surface including protection against pathogens, immunoregulation and prevention of desiccation. In the present study, we analyze the N-linked glycome of tear fluid from patients affected by vernal (VKC) and atopic keratoconjunctivitis (AKC) in order to identify potential biomarkers for these diseases. To date, no specific laboratory test are suitable for VKC and AKC diagnosis and monitoring.
Tear samples from 23 VKC patients, 7 AKC patients and 11 control subjects were diluted 1/10 in a denaturant solution, reduced, alkylated and treated with N-glycosidase F (PNGase F), an enzyme which specifically deglycosylates N-glycoproteins. Released N-glycans were purified, chemically derivate by permethylation and analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS and MALDI TOF/TOF MS/MS). The eligibility of mass spectrometry for the study of glycosylation is due to its high sensibility and ability to analyze complex mixtures of glycans from biological samples.
Our approach allowed to identify more than 150 complex N-glycans, including biantennary, triantennary, tetraantennary and bisecting species. Highly fucosylated structures were also found. Data analysis showed changes in terms of relative intensities for some structures. In VKC, the most significant peaks were related to mass to charge ratios 1906.7 and 2592.3, corresponding to a bisecting and to a N-glycan structure bearing two Lewis-X epitope, respectively.In AKC the most intense peak is found at m/z 2792.4, and corresponds to the well-known biantennary, disialylated N-glycan. Each structure reveals significant differences between healthy and pathological conditions, with major variations involving bisecting and hyperfucosylated glycoforms. Structures were confirmed in detail by MS/MS analysis.
MALDI-TOF-MS is a valuable technique for biomarker detection and characterization of defective glycan structures. Identified peaks could be used as potential biomarkers for VKC and AKC. Further structural characterization of the disease-associated N-glycans may confirm these preliminary results and allow to discover additional biomarkers.
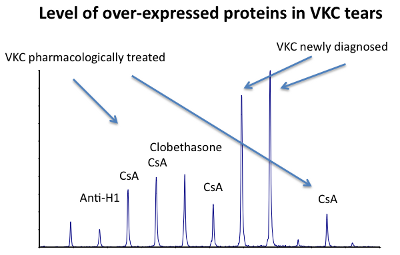
12) Identification of human tear fluid biomarkers in vernal keratoconjunctivitis using iTRAQ quantitative proteomics
A.Leonardi, A.Palmigiano, E.Mazzola, A.Messina, E.Milazzo, M.Bortolotti, D.Garozzo
Allergy
69(2),
254-260
- 2014
DOI:
https://doi.org/10.1111/all.12331
BACKGROUND: Understanding and treating vernal keratoconjunctivitis (VKC) has been a challenge because the pathogenesis is unclear and antiallergic therapy often unsuccessful. The aim of the study was to analyze peptide profiles in human tears using mass spectrometry to elucidate compositional differences between healthy subjects and patients affected by VKC.
METHODS: Tears were collected from healthy subjects and VKC patients. Digested samples were treated with iTRAQ (isobaric tag for relative and absolute quantitation). Separation of tryptic peptides was realized using a MicroHPLC interfaced with a microfraction collector. MS and MS/MS mass spectra were performed using a MALDI TOF/TOF 4800 Applied Biosystem spectrometer. Protein Pilot
TM software with Paragon
TM algorithm v4.1.46 or GPS
TM with Mascot engine was used as search engines with SwissProt or IPI human as the databases.
RESULTS: A significant number of peptides were examined, and 78 proteins were successfully identified. In all VKC samples, levels of serum albumin, transferrin, and hemopexin were found up to 100 times higher than control tear levels and correlated to the severity of disease. Hemopexin, transferrin, mammaglobin B, and secretoglobin 1D were found significantly over-expressed in VKC samples compared with the control samples. Tear samples from patients treated with topical cyclosporine or corticosteroids showed a dramatic reduction in these protein levels.
CONCLUSIONS:LC MALDI MS and isobaric tag for relative and absolute quantitation technique may be useful in the quantitative and qualitative characterization of the peptidoma of human tears. These techniques may identify target proteins to be used in the diagnosis and management of VKC and other inflammatory ocular surface conditions.
13) β-Amyloid Monomers Are Neuroprotective
M.L.Giuffrida, F.Caraci, B.Pignataro, S.Cataldo, P.De Bona, V.Bruno, G.Molinaro, G.Pappalardo, A.Messina, A.Palmigiano, D.Garozzo, F.Nicoletti, E.Rizzarelli, A.Copani
Journal of Neuroscience
29(34),
10582-10587
- 2009
The 42-aa-long β-amyloid protein--Aβ1-42--is thought to play a central role in the pathogenesis of Alzheimer’s disease (AD) (Walsh and Selkoe, 2007). Data from AD brain (Shankar et al., 2008), transgenic APP (amyloid precursor protein)-overexpressing mice (Lesné et al., 2006), and neuronal cultures treated with synthetic Aβ peptides (Lambert et al., 1998) indicate that self-association of Aβ1-42 monomers into soluble oligomers is required for neurotoxicity. The function of monomeric Aβ1-42 is unknown. The evidence that Aβ1-42 is present in the brain and CSF of normal individuals suggests that the peptide is physiologically active (Shoji, 2002). Here we show that synthetic Aβ1-42 monomers support the survival of developing neurons under conditions of trophic deprivation and protect mature neurons against excitotoxic death, a process that contributes to the overall neurodegeneration associated with AD. The neuroprotective action of Aβ1-42 monomers was mediated by the activation of the PI-3-K (phosphatidylinositol-3-kinase) pathway, and involved the stimulation of IGF-1 (insulin-like growth factor-1) receptors and/or other receptors of the insulin superfamily. Interestingly, monomers of Aβ1-42 carrying the Arctic mutation (E22G) associated with familiar AD (Nilsberth et al., 2001) were not neuroprotective. We suggest that pathological aggregation of Aβ1-42 may also cause neurodegeneration by depriving neurons of the protective activity of Aβ1-42 monomers. This "loss-of-function" hypothesis of neuronal death should be taken into consideration when designing therapies aimed at reducing Aβ burden.
14) Chemically modified tetranitro-oxacalix[4]arenes: synthesis and conformational preferences of tetra-N-(1-octyl)ureido-oxacalix[4]arenes
C.Capici, D.Garozzo, G.Gattuso, A.Messina, A.Notti, M.F.Parisi, I.Pisagattia, S.Pappalardo
ARKIVOC
VIII,
199-211
- 2009
Tetranitro-oxacalix[4]arenes 1–5, prepared by direct SNAr reaction of 1,5-difluoro-2,4-dinitrobenzene with the appropriate aromatic diol (pyrocatechol, resorcinol, hydroquinone, 2,7-dihydroxynaphthalene, and 4,4´-dihydroxybiphenyl), were subjected to Raney-nickel reduction to provide the corresponding tetraamino-oxacalix[4]arenes 6–10, which upon treatment with an excess of 1-octyl isocyanate were converted into the title compounds 11–15, featuring a pair of 1,3-bis-[N-(1-octyl)ureido]phenylene moieties doubly connected at their 4,6-positions by rigid spacers of varied geometry. All new oxacalix[4]arenes were characterized by MALDI-TOF spectrometry and NMR spectroscopy. 1H NMR data and ab initio calculations support saddle-shaped conformations for oxacalix[4]arenes incorporating pyrocatechol, resorcinol and 2,7-dihydroxynaphthalene nucleophilic components, and boat-shaped conformations for derivatives possessing hydroquinone and 4,4´-dihydroxybiphenyl spacers.
15) Self-Assembly Dynamics of Modular Homoditopic Bis-calix[5]arenes and Long-Chain á,ù-Alkanediyldiammonium Components
G.Gattuso, A.Notti, A.Pappalardo, M.F.Parisi, I.Pisagatti, S.Pappalardo, D.Garozzo, A.Messina, Y.Cohen, S.Slovak
Journal of Organic Chemistry
73(18),
7280-7289
- 2008
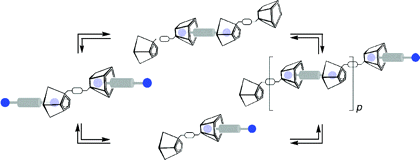
Homoditopic building blocks
1, featuring two π-rich cone-like calix[5]arene moieties connected at their narrow rims by a rigid
o-, m-, or
p-xylyl spacer in a centrosymmetric divergent arrangement, show a remarkable tendency to spontaneously and reversibly self-assemble with the complementary homoditopic α,ω-alkanediyldiammonium dipicrate guest salts
C8−C12·2Pic through iterative intermolecular inclusion events, forming supramolecular assemblies whose composition and dynamics strongly depend upon the length of the connector, the geometry of the spacer, as well as the concentration and/or molar ratios between the two components.
1H NMR spectroscopy and ESI-MS studies of
1/Cn·2Pic modular homoditopic pairs support the formation of discrete (bis)-
endo-cavity assemblies with the shorter
C8 and
C9 connectors, and/or (poly)capsular assemblies with the longer
C10−C12 components under appropriate concentrations and molar ratios (50 mM equimolar solutions).
1H NMR titration experiments and diffusion NMR studies provide clear evidence for the self-assembly dynamics of the complementary pairs here investigated.
16) Structural characterizations of lipids A by MS/MS of doubly charged ions on a hybrid linear ion trap/orbitrap mass spectrometer
A.Silipo, C.De Castro, R.Lanzetta, A.Molinaro, M.Parrilli, G.Vago, L.Sturiale, A.Messina, D.Garozzo
Journal of Mass Spectrometry
43,
478-484
- 2008
DOI:
https://doi.org/10.1002/jms.1333
Here, a new "one pot" and fast approach is described, based on electrospray ionization (ESI) of negative ions by using a hybrid linear ion trap/orbitrap mass spectrometer (LTQ/orbitrap) for MS and MS/MS analysis. By this method the distribution of the primary and secondary acyl residues of the intact lipid A is inferred by analysis of the ESI spectra measured in positive and negative mode. The analysis of these data allows an unequivocal assignment of the fatty acid distribution. This methodology was successfully tested on two different lipid A with known structures, deriving from the Agrobacterium tumefaciens and Escherichia coli lipopolysaccharides.
17) Syntheses, Structures, and Anion-Binding Properties of Two Novel Calix[2]benzo[4]pyrroles
G.Cafeo, F.H.Kohnke, A.J.P.White, D.Garozzo, A.Messina
Chemistry-A European Journal
13,
649-656
- 2007
Calix[2]benzo[4]pyrrole m-6 and p-6, each containing two dipyrromethane moieties and two m-phenylene or p-phenylene units, respectively, were readily synthesised from pyrrole, 1,3- and 1,4-bis(1,1’-dimethylhydroxymethyl)benzene, (m-4 and p-4, respectively) and acetone. Macrocycles m-6 and p-6 were tested as receptors for a selection of anions, such as acetate, dihydrogenphosphate and fluoride. The X-ray structures of m-6 and p-6 and those of the complexes m-6-F-, m-6-Cl- and m-6-CH3COO- (with an nBu4N+ counterion) were also determined.
18) Strapped Calix[2]furan[4]pyrroles, Novel Examples of Ditopic Molecular Receptors
G.Cafeo, A.Kaledkowski, F.H. Kohnke, A.Messina
Supramolecular Chemistry
18(3),
273-279
- 2006
The cyclocondensation of polyether chains functionalised with dipyrromethane units at both ends with 2,5-bis[(α-hydroxy-α,α-dimethyl)methyl]furan yield novel strapped calix[2]furan[4]pyrroles. These receptors were studied for their ability to act as ion-pair ligands towards fluoride salts.
 Top
Top





