3 book chapters
1) CSF N-Glycoproteomics Using MALDI MS Techniques in Neurodegenerative Diseases
A.Messina, A.Palmigiano, R.O.Bua, D.Romeo, R.Barone, L.Sturiale, M.Zappia, D.Garozzo
Cerebrospinal Fluid (CSF) Proteomics 2044, 255-272 - 2019
 To characterize specific N-glycan profiles of CSF in early and advanced phases of Alzheimer’s disease, as well as in lysosomal storage disorders such as Tay-Sachs disease, we set up in our lab a robust and feasible protocol by coupling bioanalytical methods and mass spectrometry analysis.
To characterize specific N-glycan profiles of CSF in early and advanced phases of Alzheimer’s disease, as well as in lysosomal storage disorders such as Tay-Sachs disease, we set up in our lab a robust and feasible protocol by coupling bioanalytical methods and mass spectrometry analysis.2) CSF N-Glycomics Using MALDI MS Techniques in Alzheimer’s Disease
A.Palmigiano, A.Messina, R.O.Bua, R.Barone, L.Sturiale, M.Zappia, D.Garozzo
Biomarkers for Alzheimer’s Disease Drug Development , 75-91 - 2018
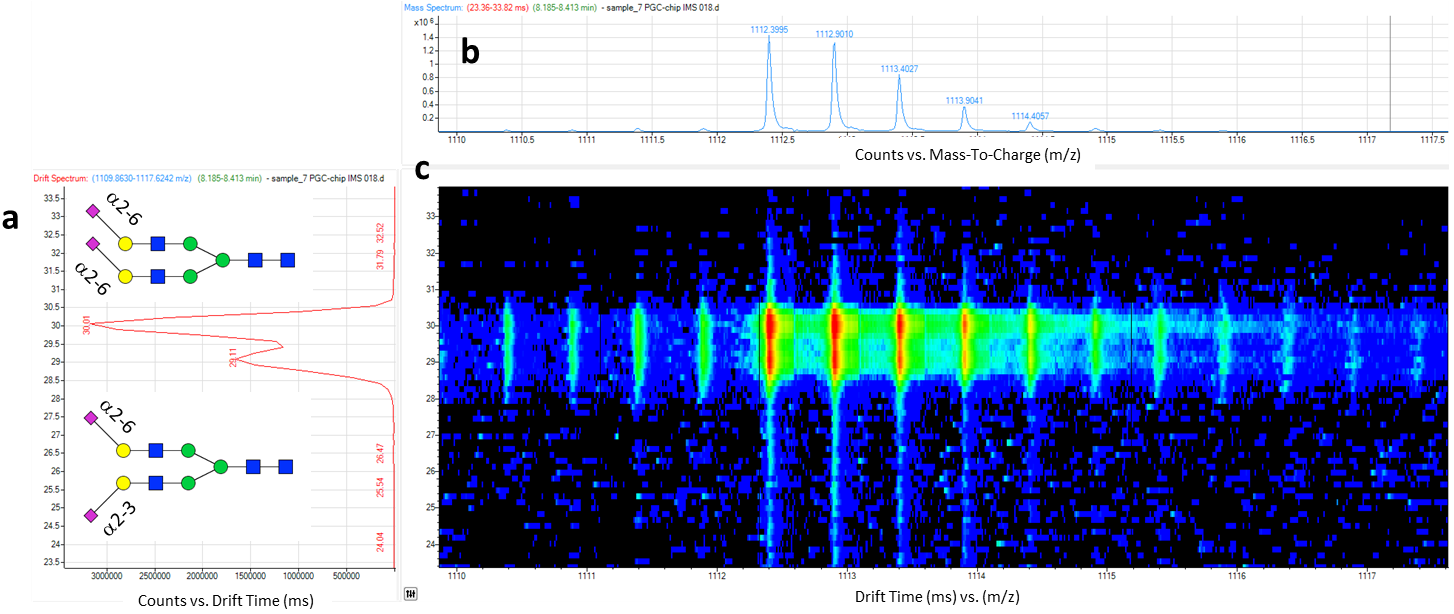
 N-glycans are released from denatured carboxymethylated glycoproteins by digestion with peptide-N-glycosidase F (PNGase F) and purified using both C18 Sep-Pak® and porous graphitized carbon (PGC) HyperSepTM HypercarbTM solid-phase extraction (SPE) cartridges. The glycan pool is subsequently permethylated to increase mass spectrometry sensitivity. Molecular assignments are performed through matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF MS) analysis considering either the protein N-inked glycosylation pathway or MALDI TOF MS/MS data. Each stage has been optimized to obtain high-quality mass spectra in reflector mode with an optimal signal-to-noise ratio up to m/z 4800. This method has been successfully adopted to associate specific N-glycome profiles to the early and the advanced phases of Alzheimer’s disease.
N-glycans are released from denatured carboxymethylated glycoproteins by digestion with peptide-N-glycosidase F (PNGase F) and purified using both C18 Sep-Pak® and porous graphitized carbon (PGC) HyperSepTM HypercarbTM solid-phase extraction (SPE) cartridges. The glycan pool is subsequently permethylated to increase mass spectrometry sensitivity. Molecular assignments are performed through matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF MS) analysis considering either the protein N-inked glycosylation pathway or MALDI TOF MS/MS data. Each stage has been optimized to obtain high-quality mass spectra in reflector mode with an optimal signal-to-noise ratio up to m/z 4800. This method has been successfully adopted to associate specific N-glycome profiles to the early and the advanced phases of Alzheimer’s disease.3) Advanced LC-MS Methods for N-Glycan Characterization
A.Palmigiano, A.Messina, L.Sturiale, D.Garozzo
Comprehensive Analytical Chemistry . Chapter 6 (ISSN 0166-526X) 79, 147-172 - 2018
 Glycosylation analysis in humans is an added value and a fundamental tool to characterize those genetic defects concerning glycan biosynthesis, such as congenital disorders of glycosylation. In addition, glycan alterations are associated with pathophysiology of major diseases as cancer and neurodegenerative dysfunctions. Concurrently, there is a growing interest of biopharmaceutical industries for the development of high-throughput methods for glycan analysis of therapeutic glycoproteins.N-linked glycans are commonly analyzed by liquid chromatography (LC) interfaced with fluorescence detection, but in the recent years a growing number of research laboratories extended the application by coupling LC with mass spectrometry (MS) techniques. Furthermore, LC-MS has become the primary device for smart high-throughput analyses.Here we report an outline of the prevailing LC-MS techniques set up for N-glycan analysis, paying special attention to N-glycomic characterization of human serum, as the most available and informative biological source for diagnosis and disease investigations.
Glycosylation analysis in humans is an added value and a fundamental tool to characterize those genetic defects concerning glycan biosynthesis, such as congenital disorders of glycosylation. In addition, glycan alterations are associated with pathophysiology of major diseases as cancer and neurodegenerative dysfunctions. Concurrently, there is a growing interest of biopharmaceutical industries for the development of high-throughput methods for glycan analysis of therapeutic glycoproteins.N-linked glycans are commonly analyzed by liquid chromatography (LC) interfaced with fluorescence detection, but in the recent years a growing number of research laboratories extended the application by coupling LC with mass spectrometry (MS) techniques. Furthermore, LC-MS has become the primary device for smart high-throughput analyses.Here we report an outline of the prevailing LC-MS techniques set up for N-glycan analysis, paying special attention to N-glycomic characterization of human serum, as the most available and informative biological source for diagnosis and disease investigations.