Dr. Filippo Samperi publications
161 publications
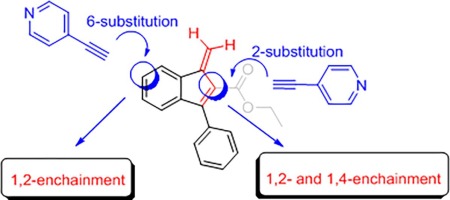
1) Spontaneous polymerization of benzofulvene monomers bearing a 4-Pyridylacetylene substituent in different positions of the benzofulvene scaffold
M.Paolino, M.Saletti, A.Reale, V.Razzano, G.Giuliani, A.Donati, C.Bonechi, G.Giorgi, G.Mercorillo, F.Samperi, W.Mroz, C.Botta, A.Cappelli
European Polymer Journal
189,
111957
- 2023
DOI:
https://doi.org/10.1016/j.eurpolymj.2023.111957
Two benzofulvene derivatives bearing a 4-pyridylacetylene substituent in different positions (i. e. 2 and 6) of the benzofulvene scaffold are designed and synthesized to evaluate the effects on the spontaneous solid-state polymerization of the presence of the same substituent in two different key positions of the 3-phenylbenzofulvene moiety. Both the benzofulvene derivatives showed the tendency to polymerize spontaneously in the consequence of solvent removal under reduced pressure without the addition of catalysts or initiators. The macromolecular structure of the stemming polymeric materials was investigated by NMR spectroscopy and MALDI-TOF mass spectrometry. Both NMR and MALDI-TOF studies confirmed the polymeric nature of the materials and suggested for the polybenzofulvene derivative bearing the 4-pyridylacetylene substituent in positions 6 a higher structural homogeneity with respect to the one bearing the same substituent in position 2. The photophysical characterization of the most homogeneous polybenzofulvene derivative led to the discovery of its outstanding hole mobility value, which was found to be around one order of magnitude higher than that previously measured for two oligothiophene-based polybenzofulvene derivatives and almost two orders of magnitude higher than that of poly(vinylcarbazole), commonly used as hole-transporter matrix. This result places the new polybenzofulvene derivative in an outstanding position as a promising material for field-effect transistor (FET) device applications.
2) Polymer Blends Based on 1-Hexadecyl-3-methyl Imidazolium 1,3-Dimethyl 5-Sulfoisophthalate Ionic Liquid: Thermo-Mechanical, Surface Morphology and Antibacterial Properties
D.Zampino, F.Samperi, M.Mancuso, T.Ferreri, L.Ferreri, S.Dattilo, E.F.Mirabella, D.C.Carbone, G.Recca, A.Scamporrino, E.Novello, C.Puglisi
Polymers
15(4),
970
- 2023
DOI:
https://doi.org/10.3390/polym15040970
In this study, antibacterial polymer blends based on Polyvinyl Chloride (PVC) and Polystyrene-Ethylene-Butylene-Styrene (SEBS), loaded with the ionic liquid (IL) 1-hexadecyl-3-methyl imidazolium 1,3-dimethyl 5-sulfoisophthalate (HdmimDMSIP) at three different concentrations (1%, 5%, and 10%), were produced. The IL/blends were characterized by their thermo-mechanical properties, surface morphology, and wettability. IL release from the blends was also evaluated. The agar diffusion method was used to test the antibacterial activity of the blends against Staphylococcus epidermidis and Escherichia coli. Results from thermal analyses showed compatibility between the IL and the PVC matrix, while phase separation in the SEBS/IL blends was observed. These results were confirmed using PY-GC MS data. SEM analyses highlighted abundant IL deposition on PVC blend film surfaces containing the IL at 5-10% concentrations, whereas the SEBS blend film surfaces showed irregular structures similar to islands of different sizes. Data on water contact angle proved that the loading of the IL into both polymer matrices induced higher wettability of the blends’ surfaces, mostly in the SEBS films. The mechanical analyses evidenced a lowering of Young’s Modulus, Tensile Stress, and Strain at Break in the SEBS blends, according to IL concentration. The PVC/IL blends showed a similar trend, but with an increase in the Strain at Break as IL concentration in the blends increased. Both PVC/IL and SEBS/IL blends displayed the best performance against Staphylococcus epidermidis, being active at low concentration (1%), whereas the antimicrobial activity against Escherichia coli was lower than that of S. epidermidis. Release data highlighted an IL dose-dependent release. These results are promising for a versatile use of these antimicrobial polymers in a variety of fields.
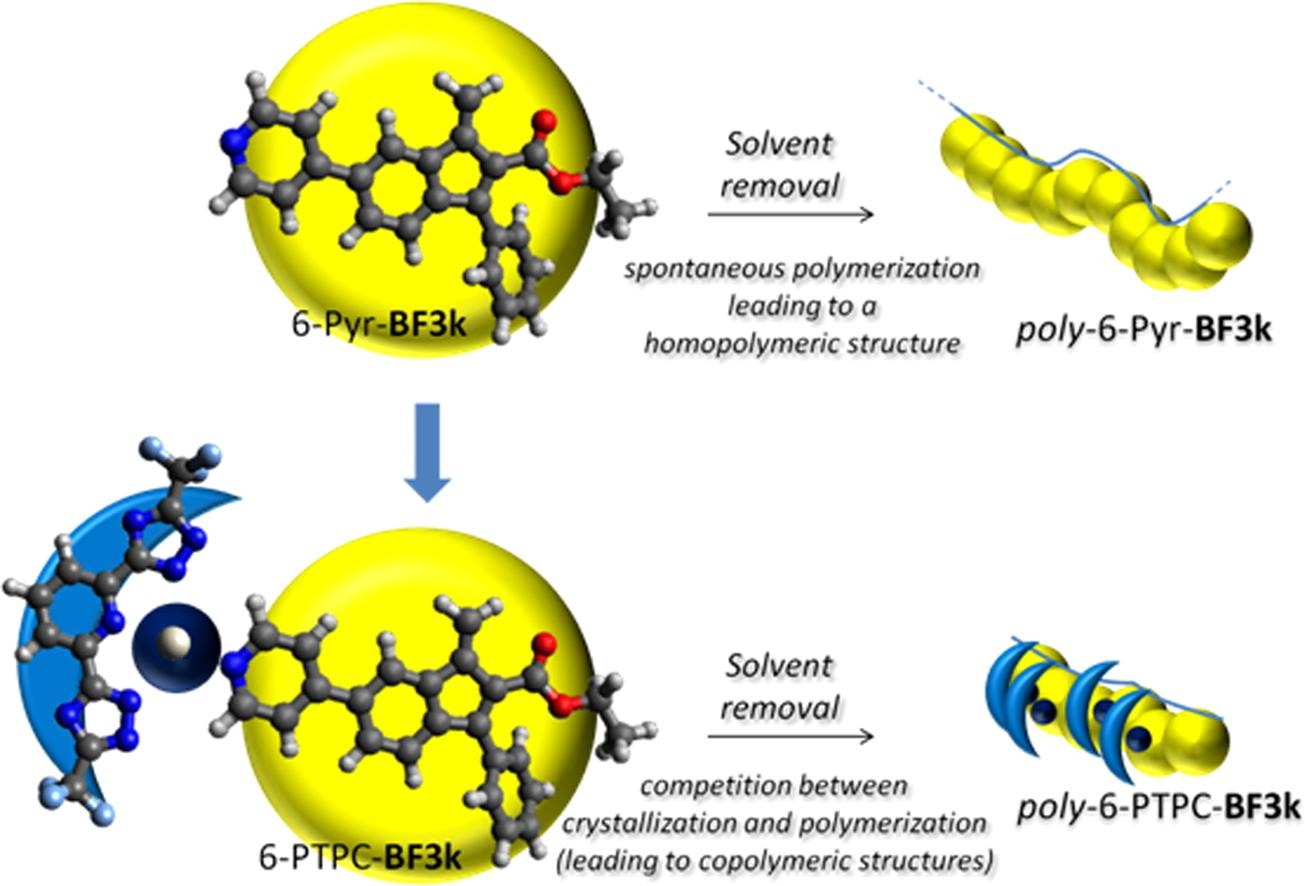
3) Spontaneous polymerization of benzofulvene derivatives bearing complexed or un-complexed pyridine rings
M.Paolino, M.Saletti, A.Reale, V.Razzano, G.Giuliani, A.Donati, C.Bonechi, G.Giorgi, A.Atrei, M.Mauro, A.Scamporrino, F.Samperi, E.Fois, G.Tabacchi, C.Botta, A.Cappelli
European Polymer Journal
169,
111137
- 2022
DOI:
https://doi.org/10.1016/j.eurpolymj.2022.111137
Benzofulvene derivatives bearing complexed and un-complexed pyridine rings are designed and synthesized to assess the effects on the spontaneous solid-state polymerization of the presence in position 6 of the 3-phenylbenzofulvene moiety of bulky substituents capable of establishing metallophilic interactions. Both the benzofulvene monomers are found to polymerize spontaneously upon solvent removal under reduced pressure in the apparent absence of catalysts or initiators. The resulting polybenzofulvene derivatives are characterized by NMR spectroscopy, MALDI-TOF mass spectrometry, and in photophysical studies.
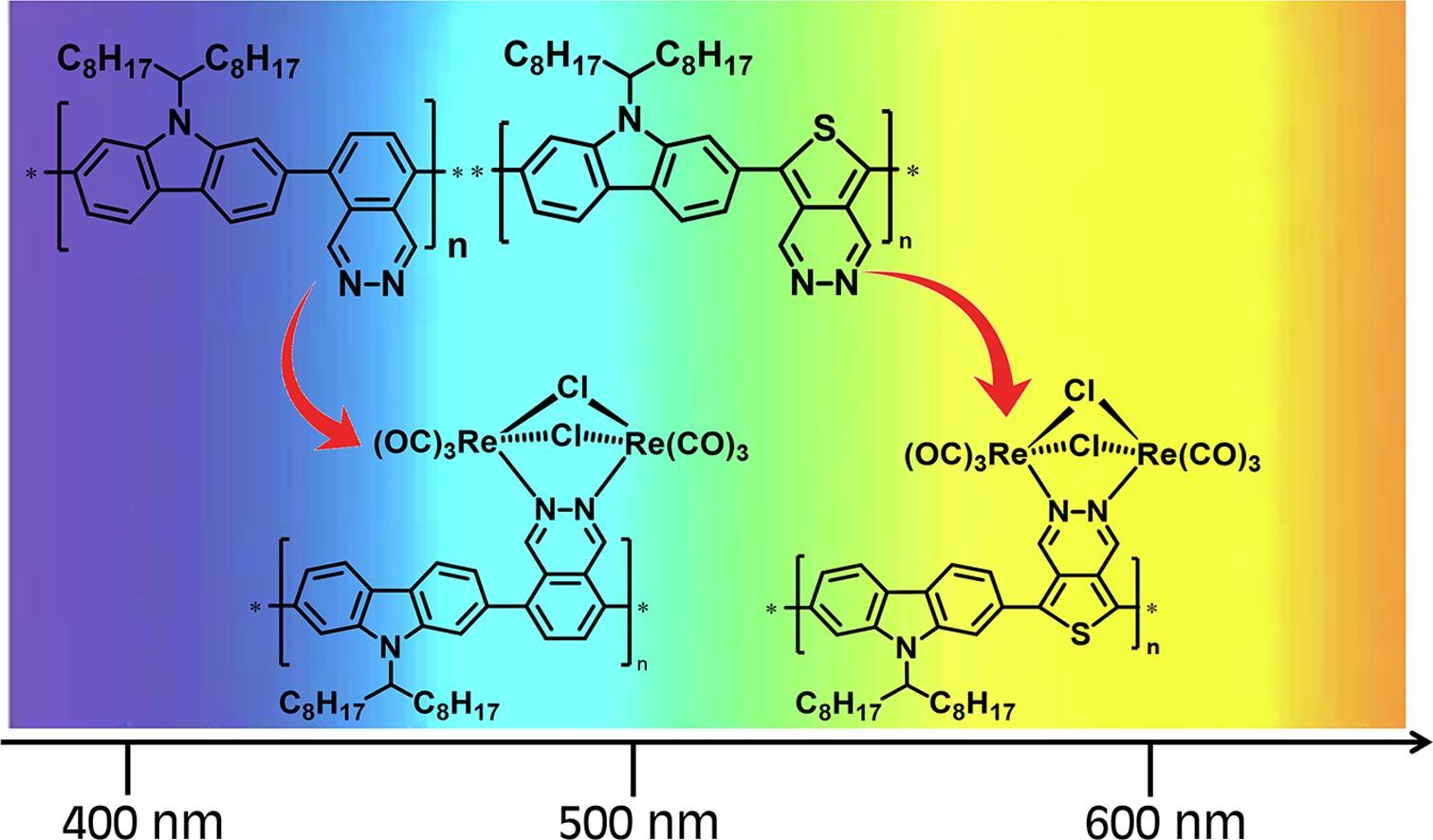
4) Carbazole-Pyridazine copolymers and their rhenium complexes: Effect of the molecular structure on the electronic properties
S.Zappia, L.Veronese, A.Fornic, S.Dattilo, F.Samperi, J.Dagare, T.M.Brown, M.Panigati, S.Destri
European Polymer Journal
168,
111095
- 2022
DOI:
https://doi.org/10.1016/j.eurpolymj.2022.111095
Two new push-pull copolymers, poly[
N-(9-heptadecanyl)carbazole-2,7-diyl-
alt-phthalazine- 5,8-diyl] (
P1) and poly[
N-(9-heptadecanyl)carbazole-2,7-diyl-
alt-thieno[3,4-d]pyridazine- 5,7-diyl]
(P2), were synthetized by means of a Suzuki polycondensation. The molecular characterization with
1H NMR and MALDI-MS showed enchainment defects in the polymer backbones depending on the synthetic conditions (catalyst and temperature). Both carbazole-carbazole and diazine-diazine homocoupling defects occurred, particularly in the case of
P2. For
P1, instead, almost homocoupling-free material was obtained. Exploiting the metal coordination capability of the pyridazine ring, corresponding dinuclear rhenium-based metallopolymers,
ReP1 and
ReP2, were also synthetized. All materials were investigated with experimental techniques (UV-vis spectroscopy, photoluminescence, cyclic voltammetry) and corroborated by theoretical studies (DFT and TD-DFT), including a qualitative evaluation of the effects of backbone defects on the electronic properties of the polymers. They were also tested in organic photovoltaic (OPV) devices. The wide energy gaps (E
g) accompanied with a low absorption coefficient for the band in the visible range reduced the harvesting capability of the copolymers. These drawbacks were partially overcome in the metallopolymers. The low device performance was mainly attributable to the low solubility of the metallopolymers in the organic solvents.
5) Characterization of VOCs and additives in Italian PET bottles and studies on potential functional aldehydes scavengers
S.Dattilo, C.Gugliuzzo, E.F.Mirabella, C.Puglisi, A.Scamporrino, D.Zampino, F.Samperi
European Food Research and Technology
248
- 2022
DOI:
https://doi.org/10.1007/s00217-022-03973-5
This study focused on characterization of Volatile Organic Compounds (VOCs) as contaminants and non-volatile additives in Italian PET bottles, also suggesting potential functional aldehydes scavengers. Several VOCs, such as acetic aldehyde (AA), butanal, 3-methyl butanal, 1,3-dioxolane, pentanal, hexanal, octanal, 5-hepten-2-one, nonanal, and decanal, were identified by Head Space-Gas Chromatography/Mass Spectrometry (HS-GC/MS) in the PET bottles used for the packaging of six Italian brands mineral waters. AA, 1,3-dioxolane, octanal, 5-hepten-2-one, nonanal, and decanal were the most abundant compounds identified. These contaminants were also identified in the PET-bottled mineral waters. Different experiments using bottle-grade PET pellets (Btlg-PET) and PET bottles’ fragments with and without the addition of epoxidized soybean oil (ESBO) or erucamide as lubricant/plasticizer additives, poly(m-xylene adipamide) (MXD6), and/or anthranilamide (2-aminobenzamide) as potential aldehydes scavengers were carried out. Mostly VOCs observed in the PET bottles analysed were identified in a neat ESBO sample. The presence of the ESBO additive in the PET-bottle fragments was also observed by matrix-assisted laser desorption/ionization time of flight mass spectrometry analysis (MALDI?TOF MS). The ESBO sub-products were not observed in the virgin btlg-PET pellets analysed by both HS-GCρMS and MALDI?TOF MS. These results suggest that the VOCs come from an ESBO additive probably loaded during the blow-moulding processes used for the manufacturing of PET bottles. Further studies established that MXD6 (1%w), an efficient oxygen scavenger, could be also used as AA scavenger even in the presence of the commonly used anthranilamide.
6) Synthesis and UV-light induced oligomerization of a benzofulvene-based neutral platinum(II) complex
M.Paolino, A.Reale, G.Magrini, V.Razzano, M.Saletti, G.Giuliani, A.Donati, F.Samperi, A.Scamporrino, M.Canetti, M.Mauro, F.Villafiorita-Monteleone, E.Foise, C.Botta, A.Cappelli
European Polymer Journal
165,
110597
- 2021
DOI:
https://doi.org/10.1016/j.eurpolymj.2021.110597
Neutral platinum(II) complex 2-PTPC-
BF3a was easily prepared from benzofulvene derivative 2-Pyr-
BF3a in order to evaluate the effects in the aggregation/polymerization behavior of a bulky substituent capable of establishing intermolecular metal-metal interactions in the close proximity of the putative polymerization center. Complex 2-PTPC-
BF3a was found to aggregate into an ordered crystalline solid-state without significant spontaneous polymerization, but the UV-light irradiation of its dispersions in chloroform produced corresponding oligomers, which were characterized by NMR spectroscopy and MALDI-TOF mass spectrometry. On the other hand, the UV-irradiation of the platinum complex in the solid-state produced different results probably depending on the aggregate architecture. Notably, the crystalline films deposited from THF solutions on quartz substrates showed weak emissions, which progressively increased upon irradiation with the formation of oligomers devoid of the aggregation-induced quenching sites that seemed to affect the emission of poly-2-PTPC-
BF3a. Finally, DFT calculations were performed on platinum complex 2-PTPC-
BF3a in the aim of rationalizing the observed photophysical features.
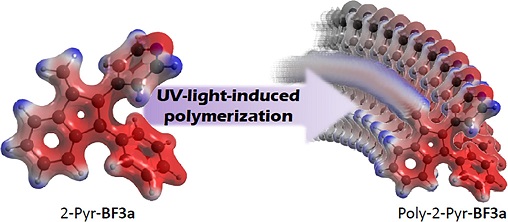
7) UV-light-induced polymerization in the amorphous solid-state of a spontaneously non-polymerizing 3-phenylbenzofulvene monomer
M.Paolino, A.Reale, G.Magrini, V.Razzano, G.Giuliani, A.Donati, G.Giorgi, F.Samperi, M.Canetti, M.Mauro, F.Villafiorita-Monteleone, E.Fois, C.Botta, A.Cappelli
European Polymer Journal
137,
109923
- 2020
DOI:
https://doi.org/10.1016/j.eurpolymj.2020.109923
Benzofulvene derivative 2-Pyr-
BF3a was designed and synthesized to evaluate the effects of a pyridine ring in position 2 of the 3-phenylbenzofulvene moiety on the spontaneous solid-state polymerization. The monomer was found to organize into an ordered crystalline solid-state without significant spontaneous polymerization, but the irradiation of amorphous film samples with UV-light produced photopolymerization. Resulting poly-2-Pyr-
BF3a was characterized by NMR spectroscopy, MALDI-TOF mass spectrometry, and photophysical studies in comparison with the corresponding monomer. Interestingly, monomer 2-Pyr-
BF3a was weakly emissive in diluted solutions, but increased its PLQY in the crystalline solid. Conversely, after photopolymerization the monomeric unit in the polymer enhanced its emission intensity in solution while in the solid state results weakly emissive. Even more interestingly, this benzofulvene monomer and its synthetic precursors
2 and
3 showed long-lived emission suggesting a phosphorescence nature of the emission process. Finally, DFT and TDDFT calculations were performed in order to rationalize the experimental data.
8) Synthesis and Characterization of Copoly(Ether Sulfone)s with Different Percentages of Diphenolic Acid Units
A.Scamporrino, C.Puglisi, A.Spina, M.S.Montaudo, D.Zampino, G.Cicala, G.Ognibene, C.Di Mauro, S.Dattilo, E.F.Mirabella, G.Recca, F.Samperi
Polymers
12(8),
1817
- 2020
DOI:
https://doi.org/10.3390/polym12081817
New functionalized Poly(ether sulfone)s having different molar ratio (10, 20, 30, 50, 70, 100 mol%) of 4,4-bis phenoxy pentanoic acid unit (diphenolic acid; DPA) units were synthesized and characterized by (
1H and
13C)-NMR, MALDI-TOF MS, FT-IR, DSC and DMA analyses. The microstructural analysis of the copolymers, obtained by
13C-NMR using an appropriate statistical model, shows a random distribution of copolymer sequences, as expected. The presence of different amount of DPA units along the polymer chains affects the chemical and physical properties of the copolymers. The Tg and the contact angle values decrease as the molar fraction of DPA units increases, whereas the hydrophilicity increases. NMR and MALDI-TOF MS analyses show that all polymer chains are almost terminated with hydroxyl and chlorine as end groups. The presence of cyclic oligomers was also observed by MALDI-TOF MS analysis.
9) Thermal Degradation Processes of Aromatic Poly(Ether Sulfone) Random Copolymers Bearing Pendant Carboxyl Groups
S.Dattilo, C.Puglisi, E.F.Mirabella, A.Spina, A.Scamporrino, D.Zampino, I.Blanco, G.Cicala, G.Ognibene, C.Di Mauro, F.Samperi
Polymers
12(8),
1810
- 2020
DOI:
https://doi.org/10.3390/polym12081810
Thermal degradation processes of poly(ether sulfone) random copolymers having different molar amount of diphenolic acid (DPA) units were studied by direct-pyrolysis/mass spectrometry, stepwise pyrolysis-gas chromatography/mass spectrometry and thermogravimetric techniques. Results highlighted that thermal degradation processes occur in the temperature range from 370 to 650 °C, yielding a char residue of 32-35 wt%, which decreases as the mol% of DPA units rises. The pyrolysis/mass spectra data allowed us to identify the thermal decomposition products and to deduce the possible thermal degradation mechanisms. Thermal degradation data suggest that the decarboxylation process of the pendant acid moiety mainly occurs in the initial step of the pyrolysis of the copolymers studied. Successively, the scission of the generated isobutyl groups occurs in the temperature range between 420 and 480 °C. Known processes involving the main chain random scission of the diphenyl sulfone and diphenyl ether groups were also observed.
10) Novel Amino Acid Assembly in the Silk Tubes of Arid-Adapted Segestriid Spiders
E.Conti, S.Dattilo, A.Scamporrino, G.Costa, F.Samperi
Journal of Chemical Ecology Journal of Chemical Ecology Journal of Chemical Ecology
46,
48-62
- 2020
DOI:
https://doi.org/10.1007/s10886-019-01127-8
We investigated in different sites inside or outside the Namib Desert the amino acids composition of the protein material forming the tube silk of Ariadna spiders. These spiders belong to the primitive Segestriidae family and spend their life inside vertical silk burrows dug within the sandy and gravelly soil of arid areas. The silks, previously purified by solubilization in hexafluoroisopropanol, were subjected to partial or total acid hydrolysis. Partial hydrolyzed samples, analyzed by mass spectrometry (matrix assisted laser desorption/ionization and electrospray), led to relevant information on the amino acid sequences in the proteins. The free amino acids formed by complete hydrolysis were derivatized with the Marfey’s reagent and characterized by electrospray mass spectrometry. The reconstruction of the amino acids highlights a homogeneous plan in the chemical structure of all the analyzed silks. Eight amino acids constituting the primary structure of the proteins were identified. Alanine and glycine are the most abundant ones, with a prevalence of alanine, constituting together at least 61% of the chemical composition of the protein material, differently from what occurs in known spidroins. High percentages of proline, serine and threonine and low percentages of leucine complete the peculiarity of these proteins. The purified silks were also characterized by Fourier-transform Infrared Spectroscopy and their thermal properties were investigated by differential scanning calorimetry. The comparison of the silk tubes among the various Namibian populations, carried out through a multivariate statistical analysis, shows significant differences in their amino acid assembly possibly due to habitat features.
11) Synthesis and characterization of a series of copolymers with different percentages of PES and DPA units
A.Scamporrino, F.Samperi, C.Puglisi, D.Zampino, S.Dattilo, A.Spina
Polychar 27 World Forum on Advanced Materials. October 14-17, 2019 - Naples, Italy
- 2019
12) Antimicrobial activity of polymeric ionic liquids
D.Zampino, F.Samperi, C.Zagni, C.Restuccia, L.Parafati, S.Dattilo, A.Scamporrino, C.Puglisi
Polychar 27 World Forum on Advanced Materials. October 14-17, 2019 - Naples, Italy
- 2019
13) Physicochemical Properties of A New PEGylated Polybenzofulvene Brush for Drug Encapsulation
M.Paolino, A.Reale, V.Razzano, G.Giuliani, A.Donati, G.Giorgi, A.C.Boccia, R.Mendichi, D.Piovani, C.Botta, L.Salvini, F.Samperi, C.Savoca, M.Licciardi, E.Paccagnini, M.Gentile, A.Cappelli
Pharmaceutics
11(9)
- 2019
DOI:
https://doi.org/10.3390/pharmaceutics11090444
A new polymer brush was synthesized by spontaneous polymerization of benzofulvene macromonomer 6-MOEG-9-T-BF3k bearing a nona(ethylene glycol) side chain linked to the 3-phenylindene scaffold by means of a triazole heterocycle. The polymer structure was studied by SEC-MALS, NMR spectroscopy, and MALDI-TOF MS techniques, and the results supported the role of oligomeric initiatory species in the spontaneous polymerization of polybenzofulvene derivatives. The aggregation features of high molecular weight poly-6-MOEG-9-T-BF3k-FE were investigated by pyrene fluorescence analysis, dynamic light scattering studies, and transmission electron microscopy, which suggested a tendency towards the formation of spherical objects showing dimensions in the range of 20-200 nm. Moreover, poly-6-MOEG-9-T-BF3k-FE showed an interesting cytocompatibility in the whole concentration range tested that, besides its aggregation features, makes this polybenzofulvene brush a good polymer candidate for nanoencapsulation and delivery of drug molecules. Finally, the photo-physical features of poly-6-MOEG-9-T-BF3k-FE could allow the biodistribution of the resulting drug delivery systems to be monitored by fluorescence microscopy techniques.
14) Copolyethersulfones with pendant carboxylic groups for metal ions absorption
F.Samperi, A.Spina, S.Dattilo, E.F.Mirabella, A.Scamporrino, D.Zampino, C.Puglisi
Eurofillers Polymerblends - Palermo (Italy) April 23-26, 2019
- 2019
Aromatic thermoplastic poly(ether-sulfone)s (PES) have reached a remarkable scientific and industrial interests owing to their excellent mechanical, chemical and thermal stabilities (1,2). This class of polymers have found application as adhesives for metal-to-metal bonds, membranes for separating gases and solids from solution or isolation of proteins, matrices for fibre rein-forced composites and as toughening agents for thermosetting resins. It has been reported in the literature that a wide spectrum of thermal, chemical and mechanical properties and also morphological behaviour, can be obtained by varying the type of monomer units in the corresponding copolymers or by derivatization of preformed commercial PESs (1,2). In the present study the chelating properties of some functionalized PES copolymers against heavy metal ions will be investigated as a function of the composition of the functional monomers.
15) Poly(styrene/styrene sulfonate)/ionic liquid copolymers with antimicrobial activity
C.Puglisi, F.Samperi, C.Zagni, C.Restuccia, L.Parafati, S.Dattilo, A.Scamporrino, D.Zampino
Eurofillers Polymerblends - Palermo (Italy) April 23-26, 2019
- 2019
Ionic liquids (ILs) are molten salts at temperature below 100°C, containing of an organic cation and an anionic portion of various nature. Their structure as well as their chemical-physical properties, such as low vapor tension, high polarity, electrical conductivity and non-flammability, make them particularly versatile and interesting (1). It was reported that imidazole-based ILs display antimicrobial activity comparable to that of commercial antimicrobial agents. Their biocidal activity is due to the length of the alkyl chain of the cation, to the type of substituted functional groups and their position in the imidazole ring (2). Recent studies have concerned the synthesis of different poly-ionic liquids (PILs), in which the cation or anion portion has a polymeric structure with interesting characteristics such as high ionic conductivity and thermal stability.
The aim of this study was the synthesis of ILs and PILs with antimicrobial activity.
16) Polymeric ionic liquids with antimicrobial activity
C.Zagni, F.Samperi, C.Restuccia, L.Parafati, S.Dattilo, C.Puglisi, D.Zampino
Frontiers in Polymer Science 5-8 May 2019, Budapest (Hungary)
- 2019
Polymeric ionic liquids (PILs) bearing imidazolium group have been extensively investigated as antimicrobial compounds due to their broad spectrum of activity and because do not easily induce bacterial resistance wìth low toxicity toward mammalian cells. With the aim to develop new antimicrobial compounds, we synthesized new ionic and polyionic liquids by metathesis reaction of 1-hexadecyl-3-methylimidazolium bromide, previously prepared by alkylation of 3-methylimidazole with 1-bromohexadecane, with different anions. The following compounds have been synthesized: 1-hexadecyl-3-methylimidazolium dimethyl-5-sulfoisophtalate (HDmim+
DMSIP-), 1-hexadecyl-3-methylimidazolium styrene sulfonate (HDmim+ SS-), 1-hexadecyl-3-methylimidazolium
poly(styrene-styrene sulfonate 75:25) (HDmim+ PSS- 75:25) and 1-hexadecyl-3-methylimidazolium poly(styrene-styrene sulfonate 90:10) (HOmim+PSS- 90:10). In order to obtain PILs, two copolymers have been produced by reaction of styrene and sulfonate styrene mixed at different molar ratios (75:25 and 90:10) and then used as anions for the synthesis of polyionic liquids HDmim+ PSS- 75:25 and HDmim+ PSS- 90:10. All the synthesized compounds have been characterized by 1HNMR, MALDI, DSC and TGA analysis. The antimicrobial activity of cotton gauzes loaded with different concentrations of ionic and polyionic liquids was investigated by disk diffusion method. The synthesìzed compounds induced a good antimicrobial activity against both Gram positive (Listeria innocua and Bacillus subtilis) and Gram negatìve (Pseudomonas fluorescens) bacterial pathogens, thus resulting promising antimicrobial agents for future applications in different fields (medical, alimentary, textile, etc.).
17) Synthesis and characterization of copolyethersulfones with pendant carboxylic groups
A.Spina, S.Dattilo, E.F.Mirabella, C.Puglisi, F.Samperi, A.Scamporrlno, D.Zampino
Frontiers in Polymer Science 5-8 May 2019, Budapest (Hungary)
- 2019
Polyethersulfone based copolymers containing different percentages of 4,4'-bis(4-hydroxyphenyl)pentanoic acid (DPA) were synthesized and investigated in order to preparare membranes for removing heavy metals. The copolymers obtained contain 10, 20, 30, 50, 70% mol of DPA and were prepared by nucleophilic polycondensation reaction between DPA and 4,4’-sulfonyldiphenol (DHDPS) wlth 4,4’-dichlorodiphenyl sulfone (DCDPS). Chemical properties and thermal stability have been studied with particular attention to the influence of the diphenolìc acid moiety on the polyethersufone. The functional carboxylic group of the DPA unit of the PES-DPA copolymers was also used for the insertion of telechelic oligomers of polyethersulfone sulphonated (S-PES) with controlled degree of polymerization to synthesize grafted sulfonated copolymers with a comb structure and improved properties in terms of proton conductivity. The proposed idea is to develop filtration rnembranes with selective properties against heavy metal ions; preliminary tests were carried out in aqueous solutions containing known percentages of heavy metals (Pb, Cr, Cu, Cd, Zn,V). Copolymers were characterized by 1H-NMR spectrometry, MALDI-TOF mass spectrometry, FTIR, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The chemlcal-structural characterization by 1H-NMR and MALDI-TOF MS analysìs confirms the formation of macromolecular chaìns terminated with OH and CI functional groups. TGA analysis shows high thermal stability of the polymers, higher in the samples containing lower percentages of DPA. Preliminary studies revealed the ability of PES-DPA copolymers to adsorb some heavy metals in a selective manner, expecially Cr and Pb in aqueous solution.
18) Liquidi ionici polimerici ad attività antimicrobica
D.Zampino, C.Zagni, F.Samperi, C.Restuccia, L.Parafati, S.Dattilo, C.Puglisi
AIM2018 -XXIII Convegno Nazionale dell’Associazione Italiana di Scienza e Tecnologia delle Macromolecole, Catania 9-12 Settembre 2018
- 2018
I liquidi ionici (ILs) sono composti costituiti da una porzione cationica organica ed una anionica di varia natura. La loro struttura così come le loro proprietà chimico fisiche, quali bassa tensione di vapore, elevata polarità, conduttività elettrica e noninfiammabilità, li rendono particolarmente versatili ed interessanti [1]. E’ stato osservato che ILs a base di imidazolo mostrano un’attività antimicrobica paragonabile a quella di agenti antimicrobici commerciali come il Triclosano e che la loro attività biocida è principalmente dovuta alla lunghezza della catena alchilica del catione, al tipo di gruppi funzionali sostituiti ed alla loro posizione nell’anello imidazolico [2]. Recentemente, sono stati sintetizzati diversi tipi di liquidi poli-ionici (PILs), in cui il catione o l’anione presentano una struttura polimerica con interessanti caratteristiche quali elevate conduttività ionica e stabilità termica. Scopo di questo studio è stato la realizzazione di fibre di cotone antimicrobiche ottenute additivando a garze di cotone liquidi ionici e poli-ionici di sintesi. Sono stati sintetizzati due liquidi ionici: l’1-metil-3-esadecil imidazolo dimetilsulfoisoftalato (EDmim+ DMSIP-) e l’1-metil-3-esadecil imidazolo stirene solfonato (EDmim+ SS-). Inoltre, sono stati sintetizzati due copolimeri salificati di stirene e stirene solfonato (PSS), a diversi rapporti molari, che sono stati utilizzati come anioni per la realizzazione dei PILs 1-metil-3-esadecil imidazolo polistirenestirene solfonato 75:25 (EDmim+ PSS- 75:25) e 1-metil-3-esadecil imidazolo polistirene-stirene solfonato 90:10 (EDmim+ PSS- 90:10). La scelta di utilizzare ILs e PILs è stata effettuata per verificare eventuali differenze nel rilascio dell’agente antimicrobico dalle fibre di cotone. In particolare, si è cercato di determinare l’influenza del diverso tipo di anione (ILs) e del diverso peso molecolare dei composti (ILs vs PILs). L’attività antimicrobica delle fibre di cotone additivate è stata testata nei confronti di batteri Gram positivi (Listeria innocua e Bacillus subtilis) e Gram negativi (Pseudomonas fluorescens) attraverso il metodo di diffusione in piastra.
19) Sintesi e caratterizzazione di copolieteresolfone aventi
gruppi acidi pendenti
A.Spina, S.Battiato, S.Dattilo, C.Puglisi, F.Samperi, D.Zampino
AIM2018 -XXIII Convegno Nazionale dell’Associazione Italiana di Scienza e Tecnologia delle Macromolecole, Catania 9-12 Settembre 2018
- 2018
I poli(etere solfone) (PES) sono polimeri termoplastici ad elevate prestazioni con elevata temperatura di transizione vetrosa (Tg), elevata stabilità termica, ottima resistenza all’idrolisi ed all’ossidazione e interessanti proprietà meccaniche
dal punto di vista ingegneristico [1-4]. A temperatura ambiente si presentano come resine dure e rigide. La loro resistenza al calore ed alla fiamma, nonchè a condizioni di esposizione prolungata a
umidità e vapore, è di gran lunga superiore a
quella delle plastiche convenzionali: sopportano temperature fino a 200°C senza mostrare significative variazioni dimensionali o deterioramento fisico anche nel lungo termine (fino a 20 anni).La loro
diffusione è ancora limitata a causa dell’elevato
costo delle materie prime e dei loro processi di lavorazione. Negli ultimi decenni sono stati prodotti e commercializzati un gran numero di PES aromatici per gli scopi più vari: come componenti per
isolanti elettrici, utensili da cucina e dispositivi medicali, per applicazione in sensori elettrochimici e membrane a scambio protonico per celle a combustibile o, ancora, come agenti tenacizzanti di resine termoindurenti e adesivi per strati metallici. I PES si prestano per la produzione di membrane con proprietà riproducibili e dimensioni dei pori controllabili fino a 40 nanometri. Le membrane esclusivamente a base di polisolfoni sono tipicamente idrofobiche e quindi soggette a scarsa bagnabilità; variando la composizione ed, in particolare, funzionalizzando con gruppi maggiormente idrofilici possiamo ottenere migliorate proprietà.
20) Densely PEGylated Polybenzofulvene Brushes for Potential Applications in Drug Encapsulation
M.Paolino, G.Grisci, F.Castriconi, A.Reale, G.Giuliani, A.Donati, C.Bonechi, G.Giorgi, R.Mendichi, D.Piovani, A.C.Boccia, M.Canetti, F.Samperi, S.Dattilo, C.Scialabba, M.Licciardi, E.Paccagnini, M.Gentile, Andrea Cappelli
Pharmaceutics
10(4),
234
- 2018
The technique of grafting side chains onto a linear polymeric backbone is commonly used to confer to the new polymeric material with desired properties, such as tunable solubility, ionic charge, biocompatibility, or specific interactions with biological systems. In this paper, two new polybenzofulvene backbones were assembled by spontaneous polymerization of the appropriate benzofulvene monomers (4,6-PO-BF3k and 4',6-PO-BF3k) bearing two clickable propargyloxy groups in different positions of the 3-phenylindene scaffold. Poly-4,6-PO-BF3k and poly-4',6-PO-BF3k were grafted with monomethyl oligo(ethylene glycol) (MOEG) to prepare two new polybenzofulvene brushes (i.e., poly-4,6-MOEG-9-TM-BF3k and poly-4',6-MOEG-9-TM-BF3k) by means of a "grafting onto" approach, that were characterized from the point of view of their macromolecular features, aggregation liability, and in a preliminary evaluation of biocompatibility. The obtained results make these PEGylated polybenzofulvene brushes (PPBFB) derivatives potentially useful as nanocarriers for nanoencapsulation and delivery of drug molecules.
21) Poly-histidine grafting leading to fishbone-like architectures
V.Razzano, M.Paolino, A.Reale, G.Giuliani, A.Donati, G.Giorgi, R.Artusi, G.Caselli, M.Visintin, F.Makovec, S.Battiato, F.Samperi, F.Villafiorita-Monteleone, C.Botta, A.Cappelli
RSC Advances
16,
8638-8656
- 2018
A small series of Morita-Baylis-Hillman adduct (MBHA) derivatives was synthesized and made to react with imidazole,
N-acetylhistidine, and
N-acetylhexahistidine as models of poly-histidine derivatives. Intriguingly, the reaction of MBHA derivatives
1a and
b with imidazole in acetonitrile-phosphate buffered saline (PBS) gave the imidazolium salt biadducts
3a and
b as the main reaction products. These results were confirmed by experiments performed with
Nacetylhistidine and
1b and suggested the possible occurrence of these structures in the products of poly-histidine labeling with MBHA derivatives
1a and
b. These compounds were then transformed into the corresponding water-soluble derivatives
1c-e by introducing oligo(ethylene glycol) chains and their reactivity was evaluated in preliminary experiments with imidazole and then with
Nacetylhexahistidine in PBS. The structure of polymeric materials
Ac-His-6-MBHA-1d and
Ac-His-6-MBHA-1e obtained using ten-fold excesses of compounds
1d and
e was investigated using mass spectrometry, NMR spectroscopy, and photophysical studies, which suggested the presence of biadduct residues in both polymeric materials. These results provide the basis for the preparation of fishbone-like polymer brushes, the characterization of their properties, and the exploration of their potential applications in different fields of science such as
in vivo fluorogenic labeling, fluorescence microscopy, protein PEGylation, up to the production of smart materials and biosensors.
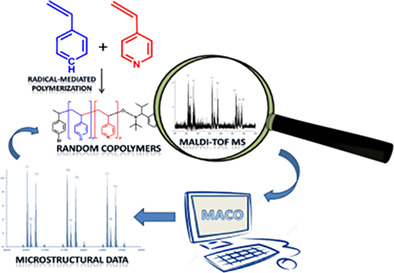
22) An innovative approach for the chemical structural characterization of poly(styrene 4-vinylpyridine) copolymers by matrix-assisted laser desorption/ionization time of flight mass spectrometry
M.S.Montaudo, C.Puglisi, S.Battiato, S.Zappia, S.Destri, F.Samperi
Journal of Applied Polymer Science
135,
46976
- 2018
Poly(styrene-co-4-vinylpyridine) random copolymers with different molar composition were synthesized by nitroxide-mediated controlled-radical polymerization using 2,2,5-trimethyl-4-phenyl-3-azahexane-3-nitroxide (TIPNO) as a mediator. We record the matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) spectra under various conditions, and we find (at last) that they show mostly intact ions [using 2(-4-hydroxyphenylazo-)benzoic acid as MALDI matrix]. Spectra are highly resolved, and thus they allow for the determination of all end-groups, even some less-abundant ones. Spectra are dominated by intact "dormant" copolymer chains terminated with TIPNO at one end and with (4-Bromo-phenyl)ethyl group (starting fragment) at the other one. Applying the mass analysis of copolymers (MACO) statistical model to the spectra, we show that the MACO/MALDI-TOF mass spectrometry (MS) analysis can be successfully applied to copolymers having a difference between the mass of the comonomers as small as 1 g mol
-1 (the styrene and 4-vinylpyridine units are 104.15 and 105.15 g/mol, respectively), which results in overlapping isotopic patterns. The results are accurate: chemical composition evaluated by means of MS agrees with that calculated by
1H-nuclear magnetic resonance, for all copolymers investigated. This analytical method allows to extract detailed information on the composition of the copolymer samples and their structure. Glass transition temperatures of copolymers were also determined by differential scanning calorimetry.
23) Hyaluronan-based graft copolymers bearing aggregation-induced emission fluorogens
A.Cappelli, M.Paolino, A.Reale, V.Razzano, G.Grisci, G.Giuliani, A.Donati, C.Bonechi, S.Lamponi, R.Mendichi, S.Battiato, F.Samperi, F.Makovec, M.Licciardi, L.Depau, Ch.Botta
Royal Society of Chemistry
8,
5864-5881
- 2018
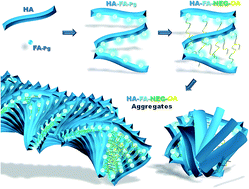
In order to develop a technology platform based on two natural compounds from biorenewable resources, a short series of hyaluronan (
HA) copolymers grafted with propargylated ferulic acid (
HA-FA-Pg) were designed and synthesized to show different grafting degree values and their optical properties were characterized in comparison with reference compounds containing the same ferulate fluorophore. Interestingly, these studies revealed that the ferulate fluorophore was quite sensitive to the restriction of intramolecular motion and its introduction into the rigid
HA backbone, as in
HA-FA-Pg graft copolymers, led to higher photoluminescence quantum yield values than those obtained with the isolated fluorophore. Thus, the propargyl groups of
HA-FA-Pg derivatives were exploited in the coupling with oleic acid through a biocompatible nona(ethylene glycol) spacer as an example of the possible applications of this technology platform. The resulting
HA-FA-NEG-OA materials showed self-assembling capabilities in aqueous environment. Furthermore,
HA-FA-NEG-OA derivatives have been shown to interact with phospholipid bilayers both in liposomes and living cells, retaining their fluorogenic properties and showing a high degree of cytocompatibility and for this reason they were proposed as potential biocompatible self-assembled aggregates forming new materials for biomedical applications.
24) Exchange Reaction Mechanisms in the reactive Extrusion of Condensation Polymers
C.Puglisi, F.Samperi
Reactive Extrusion: Principles and Applications . (Editors by Di Günter Beyer and Christian Hopmann)
Chapter 6,
135
- 2017
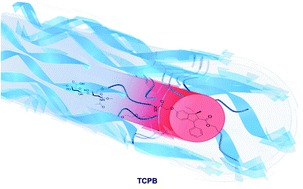
25) Hyaluronan-coated polybenzofulvene brushes as biomimetic materials
A.Cappelli, M.Paolino, G.Grisci, V.Razzano, G.Giuliani, A.Donati, C.Bonechi, R.Mendichi, S.Battiato, F.Samperi, C.Scialabba, G.Giammona, F.Makovec, M.Licciardi
Polymer Chemistry
7,
6529-6544
- 2016
Hyaluronic acid (
HA) forms pericellular coats in many cell types that are involved in the early stages of cell adhesion by interacting with the CD44 receptor. Based on the largely recognized overexpression of the CD44 receptor in tumor tissues, a polybenzofulvene molecular brush has been enveloped into hyaluronan shells to obtain a tri-component polymer brush (
TCPB) composed of intrinsically fluorescent backbones bearing nona(ethylene glycol) arms terminated with low molecular weight
HA macromolecules. The nanoaggregates obtained in
TCPB water dispersions were characterized on the basis of dimensions, zeta potential, and in vitro cell toxicity. This biomimetic multifunctional material bearing
HA on the surface of its cylindrical brush architecture showed promising prerequisites for the preparation of nanostructured drug delivery systems.
26) A novel hybrid linear-hyperbranched poly(butylene adipate) copolymer as an epoxy resin modifier with toughening effect
F.Shiravand, L.Ascione, P.Persico, C.Carfagna, Th.Brocks, M.O.Hilário Cioffi, C.Puglisi, F.Samperi, V.Ambrogi
Polymer International
65,
308-319
- 2016
A study was carried out on the effect of a hybrid linear-hyperbranched poly(butylene adipate) copolymer on the properties of a commercial epoxy resin. First, the synthesis of the hyperbranched systems was optimized. These systems were obtained by reacting linear oligomers with 1,1,1-tris(hydroxymethyl)propane used as branching agent and varying the reaction times from 16 to 44 h. The synthesized samples were characterized through matrix-assisted laser desorption ionization time-of-flight mass spectrometry, differential scanning calorimetry and thermogravimetric analysis. Results showed that for reaction times of 30 h a highly branched system, namely 5HB30, was obtained. This system was chosen as toughening agent for a commercial high-performance epoxy resin. A kinetics analysis of epoxy/5HB30 blends indicated that the hyperbranched system had no accelerator or catalytic effect on the crosslinking reaction in the resin. Furthermore, it was demonstrated that 5HB30 acted as an excellent toughening agent, increasing significantly impact resistance up to 90% with respect to neat epoxy resin. The toughness behaviours of epoxy-based blends were explained by investigating the fracture surface after impact tests through scanning electron microscopy before and after solvent etching. It was observed that the globular-like hyperbranch-rich domains, dispersed throughout the continuous epoxy resin, were able to absorb the impact energy without affecting thermal stability.
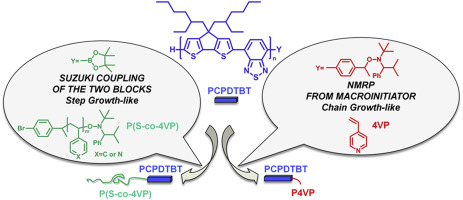
27) Characterization of amphiphilic block-copolymers constituted of a low band gap rigid segment (PCPDTBT) and P4VP based coil block synthesized by two different strategies
S.Zappia, R.Mendichi, S.Battiato, G.Scavia, R.Mastria, F.Samperi, S.Destri
Polymer
80,
245-258
- 2015
Two homologous series of rod-coil block-copolymers, composed by a low band gap rod moiety (poly[2,6-(4,4-bis-(2-ethylhexyl)-4
H-cyclopenta[2,1-
b:3.4-
b’]dithiophene)-
alt-4,7-(2,1,3-benzothiadiazole)], PCPDTBT) and a flexible poly(4-vinylpyridine) based polar block, were synthesized by two different approaches.
Step Growth-like procedure provided copolymers with longer coil, whilst copolymers with longer rod arise from
Chain Growth-like process. Both the series were deeply investigated with different analytical techniques (
1H NMR, SEC-DV, and MALDI-TOF MS) in order to prove the coupling process and elucidate the composition of the obtained materials. Chemical composition was evaluated by
1H NMR analysis. DSC heating traces of rod-coil copolymers synthesized by
Step Growth-like route present two well resolved glass transition temperatures corresponding to the rod and coil blocks. The low band-gap rod-coil block copolymers especially these with short coil can find application in hybrid solar cells.
28) Bithiophene-based polybenzofulvene derivatives with high stacking and hole mobility
A.Cappelli, V.Razzano, M.Paolino, G.Grisci, G.Giuliani, A.Donati, R.Mendichi, F.Samperi, S.Battiato, A.C.Boccia, A.Mura, G.Bongiovanni, W.Mróz, C.Botta
Polymer Chemistry
42,
7355-7486
- 2015
Four new benzofulvene derivatives bearing bithiophene chromophores at two different key positions of the phenylindene scaffold were prepared in order to evaluate the role of different chromophores in the optoelectronic features of polybenzofulvene derivatives. The results of the photophysical studies showed that the optical properties of the newly-synthesized bithiophene-functionalized polymers were affected by both the polymer enchainment and the substitution topology of the monomeric units. On the other hand, the hole-mobility appeared to be affected to a lesser extent, but the best performances were obtained in poly-6-HBT-
BF3k showing the strongest bithiophene side chain packing. This work demonstrates that the optoelectronic properties of polybenzofulvene derivatives can be optimized by a targeted chemical design such as side chain engineering.

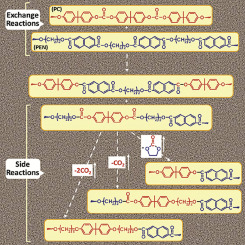
29) Reactive melt mixing of PC/PEN blend. Structural characterization of reaction products
F.Samperi, S.Battiato, G.Recca, C.Puglisi, R.Mendichi
Polymer
74,
108-123
- 2015
Chemical reactions occurring during reactive melt mixing of equimolar blend of poly(bisphenol-A carbonate) and poly(ethylene 2,6-naphthalate) at 280 °C in presence of a catalyst, were studied. Beside expected direct "inner-inner" ester-carbonate and outer-inner exchange reactions, consecutive reactions that lead to the elimination of CO
2 and ethylene carbonate from backbone, were also detected. The composition and the architecture of the formed copolymers change as the mixing time increases. Initial PC-PEN block copolymers formed at lower mixing time (2 min) evolve towards the formation of naphthalate based random copoly(ester-ether)s at reaction time higher than 45 min, owing to total elimination of carbonate units. Determination of composition and microstructure of copolymers formed was attempted by (
1H and
13C)-NMR analyses applying appropriate chemical microstructural model. Dyads and triads sequences determined here give detailed information on the change in the molar fractions of the sequences with increasing the reaction time. DSC analysis shows that block copolymer formed at 2 min mixing present two glass transition (Tg) temperatures close to those of initial homopolymers, whereas the other ones show a single Tg that changes as a function of their dyads molar compositions. Thermogravimetric analysis show that the more thermally stable copolymers are random copoly(ester-ether)s formed at higher reaction time.
30) Acetylcholinesterase-induced fluorescence turn-off of an oligothiophene-grafted quartz surface sensitive to myristoylcholine
G.Grisci, W.Mróz, U.Giovanella, K.Pagano, W. Porzio, L.Ragona, F.Samperi, S.Tomaselli, F.Galeotti, S.Destria
Journal of Materials Chemistry B
3,
4892-4903
- 2015
Conjugated polyelectrolytes (CPEs) have recently emerged as label-free materials for biosensing due to their intrinsic ability to transduce an amplified optical signal in response to interactions with different analytes. Herein, the conformational change of an anionic oligothiophene is exploited to generate a unique fluorescent response upon interaction with myristoylcholine (MyrCh). The variations observed in spectroscopic signals are explained in terms of a synergistic combination of hydrophobic and electrostatic forces involving the oligothiophene chains and MyrCh molecules, inducing the disassembling of oligothiophene chains. The enzyme acetylcholinesterase (AChE) is able to reverse this effect by catalyzing the hydrolysis of MyrCh; hence, its enzymatic activity can be monitored through the variation of fluorescence emission of the system. The oligothiophene sensing probe retains its conformational sensitivity with regard to the AChE-mediated cleavage of MyrCh upon immobilization onto a quartz substrate, which is accomplished by a "grafting onto" approach based on click chemistry. These results are encouraging for the further development of such a label-free system towards the fabrication of sensing devices that would incorporate CPEs and would be potentially useful for the specific detection of a wide range of bioanalytes.
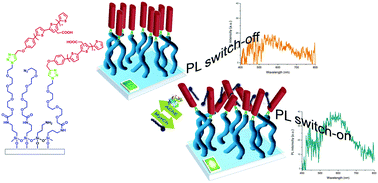
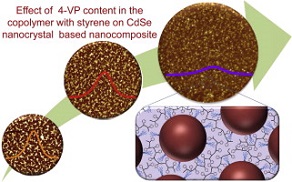
31) Segmented poly(styrene-co-vinylpyridine) as multivalent host for CdSe nanocrystal based nanocomposites
A.E.Di Mauro, M.Toscanini, D.Piovani, F.Samperi, M.L.Curria, M.Corricelli, L.De Caro, D.Siliqi, R.Comparelli, A.Agostiano, S.Destri, M.Striccoli
European Polymer Journal
60,
222-234
- 2014
Nanocomposites based on colloidal CdSe nanocrystals (NCs) and a poly(styrene-
co-4-vinylpyridine), able to specifically coordinate the NC surface, have been designed and prepared. For first time, the polymer synthesis has been performed by using 2,2,5-tri-methyl-4-phenyl-3-azahexane-3-nitroxide as a mediator, increasing the percentage of 4-vinylpyridine monomeric unit, thus obtaining a random copolymer. The nanocomposite properties have been investigated as a function of NC surface chemistry and copolymer composition, by means of spectroscopic, morphological and structural characterization techniques. An improved uniformity of NC dispersion in the nanocomposite has been found at increased percentage of 4-vinylpyridine in the copolymer. The improved NC dispersion in the nanocomposite films has been discussed in terms of the ability of the copolymer to act as a multivalent ligand. The reported results offer a valuable contribution toward the design and the fabrication of innovative nanocomposite material, formed of copolymers and colloidal NCs, specifically suited for energy conversion applications.
32) Do habitat features affect the composition of silk proteins by Namibian arid-adapted Ariadna spiders (Araneae: Segestriidae)?
E. Conti, E.Barbagallo, S.Battiato, A.Marletta, G.Costa, F.Samperi
Italian Journal of Zoology
82,
1-13
- 2014
This paper is the first to describe the silk produced by Segestriidae spiders. Field and specimen data together with webs secreted by Namibian arid-adapted Ariadna spiders were collected from different research work stations. The silks were solubilized with hexafluoroisopropanol and characterized by Fourier transform infrared (FT-IR), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and differential scanning calorimetry (DSC) techniques, in order to get data on the silk composition. FT-IR analysis confirms that proteins are the main component of the extracted materials. MALDI-TOF mass spectra of silks of three different sites (R, M and K, with three varied environmental conditions: coastal foggy area, hot central desert area and savannah) reveal the presence of low-molecular-weight (< 10,000 g/mol) proteins mostly based on glycine and alanine amino acids. Besides many peptides with identical or similar composition, other low-molar mass proteins with different compositions were also revealed in these silks. The DSC curves show that the studied silks have different melting temperature ranges. This behaviour may be due to the proteins having different molecular weight and/or different amino acid composition. The presence of small peptides with different amino acid composition could be correlated to the different habitats where the spiders live. Hypotheses linking different amino acid compositions with environmental features are suggested.
33) Reversible Polymerization Techniques Leading to π-Stacked Polymers
A.Cappelli , M.Paolino, G.Grisci, G.Giuliani, A.Donati, A.C.Boccia, F.Samperi, R.Mendichi, S.Vomero
π-Stacked Polymers and Molecules
,
51-149
- 2013
Serendipity has often played a pivotal role in research and, in harmony with this function, it has had a fundamental responsibility in the discovery of the thermoreversible spontaneous polymerization of benzofulvene derivatives.

After a decade from the discovery of poly-
BF1, the present chapter narrates the history of polybenzofulvene derivatives from the discovery to the latest developments. Now that more than 50 polymers belonging to this family have been synthesized and the relevant studies have been published in the most important journals dealing with polymer science, the spontaneous polymerization of benzofulvene monomers and the special features of the corresponding polymers appear to become a well-established topic. The chapter provides an in-depth analysis of the literature on the subject, from the preparation methods to the characterization of polybenzofulvene derivatives, the study of their properties, and the evaluation of the possible applications.
34) Combining spontaneous polymerization and click reactions for the synthesis of polymer brushes: a "grafting onto" approach.
A.Cappelli, G.Grisci, M.Paolino, F.Castriconi, G.Giuliani, A.Donati, S.Lamponi, R.Mendichi, A.C.Boccia, F.Samperi, S.Battiato, E.Paccagnini, M.Gentile, M.Licciardi, G.Giammona, S.Vomero
Chemistry-A European Journal
19(29),
9710-9721
- 2013
Two novel benzofulvene monomers bearing propargyl or allyl groups have been synthesized by means of readily accessible reactions, and were found to polymerize spontaneously by solvent removal, in the apparent absence of catalysts or initiators, to give the corresponding polybenzofulvene derivatives bearing clickable propargyl or allyl moieties. The clickable propargyl and allyl groups were exploited in appropriate click reactions to develop a powerful and versatile "grafting onto" synthetic methodology for obtaining tailored polymer brushes.
35) Role of 2-Hydroxyethyl End Group on the Thermal
Degradation of Poly(ethylene terephthalate) and
Reactive Melt Mixing of Poly(ethylene terephthalate)/
Poly(ethylene naphthalate) Blends
I.Blanco, G.Cicala, C.L.Restuccia, A.Latteri, S.Battiato, A.Scamporrino, F.Samperi
Polymer Engineering and Science
52,
2498-2505
- 2012
In an attempt to minimize the acetaldehyde formation at the processing temperatures (280-300°C) and the outer-inner transesterification reactions in the poly (ethylene terephthalate) (PET)-poly(ethylene naphthalate) (PEN) melt-mixed blends, the hydroxyl chain ends of PET were capped using benzoyl chloride. The thermal characterization of the melt-mixed PET-PEN blends at 300°C, as well as that of the corresponding homopolymers, was performed. Degradations were carried out under dynamic heating and isothermal conditions in both flowing nitrogen and static air atmosphere. The initial decomposition temperatures (Ti) were determined to draw useful information about the overall thermal stability of the studied compounds. Also, the glass transition temperature (Tg) was determined by finding data, indicating that the end-capped copolymers showed a higher degradation stability compared to the unmodified PET and, when blended with PEN, seemed to be efficient in slowing the kinetic of transesterification leading to, for a finite time, the formation of block copolymers, as determined by 1H-NMR analysis. This is strong and direct evidence that the endcapping of the -OH chain ends influences the mechanism and the kinetic of transesterification.
36) Synthesis and characterization of charge-transporting ð-stacked
polybenzofulvene derivatives
A.Cappelli, M.Paolino, G.Grisci, G.Giuliani, A.Donati, R.Mendichi, A.C.Boccia, C.Botta, W.Mroz, F.Samperi, A.Scamporrino, G.Giorgi, S.Vomero
Journal of Materials Chemistry
22,
9611-9623
- 2012
Two new benzofulvene derivatives bearing two or three methoxy substituents on the benzene ring were synthesized and induced to polymerize spontaneousl0y in order to investigate the photophysical and electronic properties of the corresponding polymers. The photophysical features of the newly synthesized polymers suggested a high degree of π-stacking both in the solid state and in diluted solutions, and the large Stokes shift was interpreted in terms of an efficient energy transfer within the excimer. Absorption and emission features in the solid state were found to be similar to those in diluted solutions and the stable
PL quantum yield was considered a promising feature with regard to the potential applications of the polymers in light emitting devices. Finally, the remarkable hole mobility shown by poly-4,5,6-MO-
BF3k along with the enhancing effect of the methoxy substituents in the charge mobility opens up new routes to the development of materials potentially useful in optoelectronics.
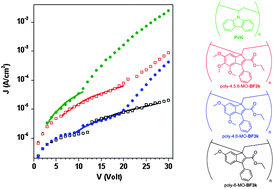
37) Combined Techniques for the Characterization of Polyfluorene Copolymers and Correlation with their Optical Properties
F.Samperi, S.Battiato, C.Puglisi, U.Giovanella, R.Mendichi, S.Destri
Macromolecules
45,
1811-1824
- 2012
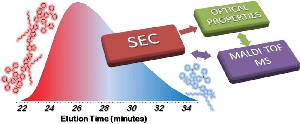
New red- and green-emitting copolymers, hereafter
core-copolymers, bearing a 4,7-bis(thiophen-2-yl)benzothiadiazole and a benzothiadiazole residue respectively as bridging
core between two identical polymeric arms were synthesized by Suzuki coupling reaction of the dibromine derivative of such chromophores and essentially borolane-ended alternating copolymers [namely
P(TPAF)] of triphenylammine disubstituted fluorene and dialkylsubstituted fluorene. All polymer samples were characterized by 1H NMR and in particular by MALDI-TOF MS. MALDI mass spectra allow the identification of many end groups of the initial blue-emitting macromers and therefore of the side reactions occurring during Suzuki polycondensation. The average molar masses were determined by two different SEC apparatus, one calibrated with conventional polystyrene narrow standards and the other with an absolute calibration curve built up by SEC/MALDI-TOF MS analysis of selected SEC fractions of polydisperse red and green
core-copolymers. MALDI mass spectra of these fractions give reliable information on their composition, which combined with their integrated area calculated from the corresponding normalized SEC curves, enable the estimation, for the first time, of the percentage of macromolecules containing the dyes composing the neat
core-copolymers. Optical characterization, performed by UV-visible absorption and photoluminescence measurements, of the same SEC fractions gives results in agreement with the different compositions determined by their MALDI mass spectra.>
38) Combined techniques for the characterization of linear hyperbranched hybrid PBA copolymers
L.Ascione, V.Ambrogi, C.Carfagna, S.Battiato, C.Puglisi, A.Scamporrino, F.Samperi
XX Convegno Italiano di Scienza e Tecnologia delle Macromolecole (AIM) TERNI 4 - 8 Settembre 2011
- 2011
Linear-hyperbranched hybrid poly(butylene adipate) copolymers (HPBA)s were synthesized through a branching reaction between the linear tailored pre-polymer terminated with methyl ester groups and different mol percents of the 1,1,1-tris(hydroxymethyl) propane (TMP) as branching agent, using the titanium(IV) isopropoxide (TIP) as catalyst, at 180 °C under vacuum for different time. All samples were characterized by NMR and MALDI-TOF Mass Spectrometry (MALDI-TOF MS). In particular, MALDI-TOF mass spectra of the unfractionated and SEC-fractionated hyperbranched (HB) samples gave information on their composition, on the end groups as well as on the TMP units present in each family of HB macromolecules. HB chains containing cyclic branches and ether bonds formed by inter-molecular trans-esterification and (intra and inter)-molecular trans-etherification side reactions, respectively, were also revealed by MALDI-TOF MS analysis. All samples were also investigated by size exclusion chromatography (SEC) and differential scanning calorimeter (DSC) tools. The average molar masses (MM) evaluated by SEC calibrated with the polystyrene (PS) narrow standards were overestimated with respect to those calculated by the SEC/MALDI-TOF MS self-calibration method, which gave reliable values. Moreover, it also showed that the hydrodynamic volume of the HPBA polymers was higher than that of the linear (PS)s with similar MM. DSC data showed that all (HPBA)s exhibited a glass transition (Tg) in the temperature range of linear poly(butylene adipate) (PBA) (-56 °C) and two melting points (Tm), whose values were dependent on the TMP content and the reaction time.
39) Synthesis and characterization of sulfonated copolyethersulphones (S-PES) having block of unsolfonated PES units
A.Scamporrino, F.Samperi, G.Cicala, R.Mendichi
XX Convegno Italiano di Scienza e Tecnologia delle Macromolecole (AIM) TERNI 4 - 8 Settembre 2011
- 2011
Polyethersulphones are a class of polyaromatics of high importance for special applications which includes toughening modifiers for epoxy resins, adhesives for metal to metal bonding, membranes for separate gaseous and solid substances and membranes for fuel cells (PEMFC). In the field of PEMFC perfluorosulfonic acid ionomers such as Nafion® and Flemion® have made a great breakthrough, and sulfonated polyethersulphones (S-PES) constitute an alternative promising class of materials owing of their high mechanical strength and high chemical, thermal and oxidative stabilities. The present contribute deals with the synthesis and characterization of some sulfonated copolyethersulphones. The synthetic approach differs from the post sulfonation approach traditionally reported in the literature, it is based on the use of sulfonated monomers which are then reacted with previously synthesized telechelic hydoxy-ended poly(ether sulphone)s. Combining the MALDI-TOF MS and 1H-NMR analyses, with SEC-Viscometry and TGA measurements, we demonstrate a powerful tool to characterize the chemical composition, end chains, degree of sulfonation and molecular mass distribution of disulfonated poly(arylene ether sulfone) copolymers
40) Combined techniques for the characterization of polyfluorene copolymers and correlaction whit their optical properties. Part 1
F.Samperi, S.Battiato, C.Puglisi, S.Destri, U.Giovanella, M.C.Pasini, R.Mendichi
XX Convegno Italiano di Scienza e Tecnologia delle Macromolecole (AIM) TERNI 4 - 8 Settembre 2011
- 2011
The energy demand is continuously growing up while the present energy sources are largely pollutant and limited. Hence, it is mandatory both to find out new alternative sources and try to reduce energy consumption. In this view the preparation of conjugated polymeric materials and their deep characterization becomes more and more important in order to correlate molecular properties with the optoelectronic ones. In this contribute polyfluorenes are considered because they are one of the most used materials for polymeric OLED fabrication. In particular these polyfluorenes allow for fabrication of white OLED which can be used for solid-state lighting and contribute to energy saving. Two series of fluorene based copolymers containing the benzothiadiazole units (Bzt; green chromophore) or the read chromophore (bis-thien-2yl)-benzothiadiazole units (T-Bzt-T) along the chains, were synthesized by Suzuki coupling reaction of tailored polyfluorene pre-polymers (PF) essentially terminated with propane boronic ester groups (PF sample) with the di-bromine derivative of the Bzt and T-Bzt-T monomers, in presence of tetrakis(triphenylphosphine) palladium ((Ph3P)4Pd) as catalyst. Two tailored PF pre-polymers constituted of alternated triphenylammino disubstituted fluorene units and dialkylsubstituted fluorine units were preliminary synthesized by Suzuki coupling reactions of equimolar ratio of 9,9-bis(4-diphenylaminophenyl)-2,7-dibromofluorene (1) and 2-(4,4,5,5-tetramethyl-1,3,2-dioxoborolane)-9,9-dioctylfluorene (2) in toluene solvent, using the same Pd catalyst, K2CO3 2 M and of a catalytic amount of Aliquat. After 6 or 10 h of reaction (depending on the run ) at 90°C, the bromine end group was quenched by adding phenylboronic acid pinacol ester in different ratios (1:1 or 1:3 with respect to the monomer equivalent) and stirring for 12 h or 18h at 90 °C in the two runs, respectively. Thus two PF samples with an weight average molar mass (Mw) of 5200 and 12700 g/mol, respectively, were obtained with the aim to better modulate the energy transfer to get white emission. These PFs were used to synthesize two series of polyfluorene based copolymers containing the green chromophore Bzt or the read chromophore one Tf-Bzt-Tf along the chains, by Suzuki coupling reaction with the di-bromine derivative of the Bzt and Tf-Bzt-Tf monomers respectively, in presence of ((Ph3P)4Pd) as catalyst. The average molar masses of all samples were calculated by SEC using THF as mobile phase, and two on-line detectors: (1) differential viscometer (DV); (2) differential refractometer (DRI model 2414). The chemical composition of the PFs and the copolymers (referred as PF-Bz and PF-(Tf-Bz-Tf)) were investigated by (1H and 13C) NMR and MALDI-TOF Mass Spectrometry tools (MALDI-TOF MS). In particular, the MALDI-TOF MS analysis of the neat polymers and copolymers and of the their narrow polydisperse fractions obtained by SEC fractionation gave peculiar information on their composition and on the side reactions occurring during the syntheses of the PF pre-polymers. The optical characterization (UV visible absorption and Photoluminescence) of the neat copolymers and of their SEC fractions were also performed and the data will be discussed in another contribute (see Combined techniques for the characterization of polyfluorene copolymers and correlaction whit their optical properties. Part 2 - Spectroscopy Measurements)
41) Characterization of Copoly(ester-amide)s From Reactive Blending of PET/MXD6 Blends in the Molten State
F.Samperi, M.S.Montaudo, S.Battiato, D.Carbone, A.Scamporrino, C.Puglisi
Advances in Polymer based Materials and Related Technologies (Capri -NA May 29th - June 1st, 2011)
- 2011
PET-MXD6 copolymers were prepared by reactive blending of equimolar PET/MXD6 blends at 285 °C for different time in presence of terephthalic acid (1 %w). Firstly, the partial hydrolysis of PET and MXD6 occurs, yielding oligomers terminated with reactive aromatic carboxyl groups. These oligomers quickly react with ester and amide inner groups producing a PET-MXD6 copolymer, that may be compatibilize the initial biphasic blend. In this homogeneous environment, the aliphatic carboxyl-terminated MCD6 chains, inactive in the initial biphasic blend, may promote the exchange reactions determining the formation of a random copolymer at longer reaction time (120 min). The progress of exchange reactions, and the microstructure of the formed copolyeteramides, versus the reaction time, was followed by (1H and 13C)-NMR analyses using a CDCl3/TFA-d/(CF3CO)2O mixture as solvent, and applying appropriate mathematical models. Dyads and triads sequences were thoroughly characterized by NMR. Block copolymers were formed at short reaction time; the average sequence lengths of each block decreases as increase the reaction time and random copolymers were formed at reaction time higher than 75 min. DSC data show that semi-crystalline block copolymers were obtained at reaction time lower than 45 min, while the other copolymers are amorphous. All PET-MXD6 copolymers shows a single Tg that changes as a function of the dyads molar composition in the copolymers. The measured Tg values match with those calculated by an proposed modified Fox equation that take into account the weight fraction of the four dyads components the PET-MXD6 copolymers. The composition of the randon copolymers was also investigated by MALDI-TOF MS analysis. PET and MXD6 were also heated with and without TA at the same processing conditions. MALDI-TOF analysis of the heated MXD6 and of the PET-MXD6 copolymers reveals also the formation of cyclopentanone ended chains, owing to the degradative reactions involving the adipic acids end groups.
42) Synthesis of PC-Ny6 block copolymers as a compatibilizers in Nylon6/Polycarbonate blends
S.Battiato, D.Carbone, A.Scamporrino, F.Samperi, C.Puglisi, A.Turturro, M.Castellano
Advances in Polymer based Materials and Related Technologies (Capri -NA May 29th - June 1st, 2011)
- 2011
PC-Ny block copolymers were prepared by reacting carboxyl-terminated Ny6 polymers (Ny6-COOH) with high molar mass PC at two different temperature (250-260 °C), for different tange time and with or without diphenylsulfone (DPSO) as solvent. The reaction produces block copolymers of the type PC-Ny6 (AB) plus a certain amount of un-converted PC degraded to lower molecular weights. The reaction products were separated by selective fractionation with solvents, obtaining three different fractions: THF soluble fraction (THF-SF), TFE soluble fraction (TFE-SF), and the TFE insoluble fraction (TFE-IF). Each fraction has been characterized by NMR and MALDI-TOF MS. All NMR spectra were recorded using CDCl3/(CF3CO)3O (90/10 v/v) mixture as solvent. The MALDI-TOF and NMR spectra of the THF-SF show that it is mainly constituted by unreacted and phenol terminated PC chains. The mass spectrum and 1H-NMR spectrum of the TFE-SF shows only the presence of typical Ny6-carboxyl terminated series of peaks. Both techniques show that all the TFE-IF were constituted of PC-Ny6 block copolymers. Two PC-Ny6 copolymer with a molar composition 80/20 and 70/30 were obtained. As expected, their DSC analysis shows that both block copolymers present two Tg values: at 47°C and 137çC for PC-Ny6 80/20 (Cop82); at 47°C and 131°C for PC-Ny6 70/30 (Copo73), due to the Ny6 and PC blocks, respectively.
Blends of Ny6/PC 80/20 (w/w) with 2% phr of one of the two copolymers, as compatibilizers, were mixed in the Brabender mixer (250°C, 30 rpm) under N2 atmosphere for 5 and 10 min. The compatibilizing action of the two block copolymers have been invrstiagted by SEM analysis. SEM micrographies show that compatibilized Ny6/PC (80/20 mol/mol) blends were obtained when were processed in presence of the PC-co-Ny6 block copolymers (2%w). Blend mixed with 2%of PC-co-Ny6 80/20 present the better mechanical properties.
43) Synthesis, Characterization, and Properties of New Phosphorus-Containing Epoxy Resins
S.Failla, P.Finocchiaro, G.A.Consiglio, F.Samperi, S.Battiato, A.Scamporrino
Phosphorus, Sulfur, and Silicon and the Related Elements
186(11),
2189-2201
- 2011
Four new phosphonate-epoxy monomers were synthesized and characterized by
1H and
31P-NMR, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), thermal gravimetric analysis (TGA), and differential scanning calorimetry (DSC) tools. Beside the expected compund, the formation of a small amounts of linear oligomers was also observed by MALDI-TOF MS. These monomers were used to prepare new phosphorus-containing epoxy resins in order to obtain potential flame retardant polymers. The thermal behavior of these new epoxy resin was studied by DSC, TGA, and direct pyrolysis mass spectrometry (DPMS). TGA and DPMS data show that either the maximum decomposition temperature (PDT) or the onset of the thermal degradation of the phosphorus-containing epoxy resins is lower than that of the corresponding nonphosphonate samples.
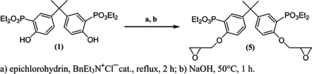
44) A click chemistry-based "grafting through" approach to the synthesis of a biorelevant polymer brush
A.Cappelli, M.Paolino, G.Grisci, G.Giuliani, A.Donati, R.Mendichi, A.C.Boccia, F.Samperi, S.Battiato, E.Paccagnini, E.Giacomello, V.Sorrentino, M.Licciardi, G.Giammona, S.Vomero
Polymer Chemistry
2,
2518
- 2011
A new biorelevant polymer brush showing a polybenzofulvene backbone was synthesized by a "grafting through" approach based on click chemistry and spontaneous polymerization reactions. The easy polymerization of the relatively complex monomer (6-MOEG-9-TM-BF3k) suggests the existence of a particularly efficient recognition process capable of pre-organizing the monomer molecules for the spontaneous polymerization. 13C-NMR spectroscopy as well as UV-vis and fluorescence spectroscopy suggested for poly-6-MOEG-9-TM-BF3k the features of a vinyl (1,2) π-stacked polymer. The new polybenzofulvene derivative was found to interact with water at room temperature to give clear water solutions, but TEM analysis demonstrated the presence of macromolecular aggregates showing dimensions larger than those suggested by SEC-MALS analysis for the isolated macromolecules. DLS studies confirmed the presence of objects showing average dimensions in the range of 200-300 nm and suggested thermoresponsive colloidal properties for poly-6-MOEG-9-TM-BF3k macromolecules. Finally, owing to its favourable absorption/emission properties and water solubility, the interaction of poly-6-MOEG-9-TM-BF3k with live cells was studied by fluorescence microscopy experiments, which revealed that the polymer brush was unable to enter live cells and alter cell morphology.
45) Combined Techniques for the Characterization of Linear-Hyperbranched
Hybrid Poly(Butylene Adipate) Copolymers
F.Samperi, S.Battiato, C.Puglisi, A.Scamporrino, V.Ambrogi, L.Ascione, C.Carfagna
Journal of Polymer Science part A: Polymer Chemistry
49,
3615-3630
- 2011
Linear-hyperbranched hybrid poly(butylene adipate) (HPBA) copolymers were synthesized through a branching reaction between the linear tailored prepolymer terminated with methyl ester groups and different mol percents of the 1,1,1-tris(hydroxymethyl) propane (TMP) as branching agent, using the titanium(IV) isopropoxide as catalyst, at 180 C under vacuum for different times. All samples were characterized by NMR and matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). In particular, MALDI-TOF mass spectra of the unfractionated and size exclusion chromatography (SEC)-fractionated hyperbranched (HB) samples gave information on their composition, on the end groups as well as on the TMP units present in each family of HB macromolecules. HB chains containing cyclic branches and ether bonds formed by intermolecular transesterification and intramolecular and intermolecular transetherification side reactions, respectively, were also revealed by MALDI-TOF MS analysis. All samples were also investigated by SEC. The average molar masses (MMs) evaluated by SEC calibrated with the polystyrene (PS) narrow standards were overestimated with respect to those calculated by the SEC/MALDI-TOF MS selfcalibration method, which gave reliable values. Moreover, it also showed that the hydrodynamic volume of the HPBA polymers was higher than that of the linear PSs with similar MMs.
46) Handbook of Engineering and Speciality Thermoplastics: Volume 3: Polyethers and Polyesters (chapter 12 "Polycarbonates")
F.Samperi, M.S.Montaudo, G.Montaudo
Sabu Thomas and Visakh P.M.
3,
493-529
- 2011
This chapter reviews the research and the most relevant progresses in polycarbonates (PC)s science and provides a comprehensive source of information on histoty, synthesis, processing and applications.

The application of different polymerization procedure of hte commercial aromatic bisphenol-A polycarbonate (referred herein as PC) and the innovative enzymatic catalysed polymerization of aliphatic polycarbonate are summarized. Due to the high engineering performance of PC polymer, an extensive section on mechanical, electrical, chemical and therma properties is included. The thermo and photo oxidative behaviours, the hydrolytic stability and the consequent modification on PC chemical structure are also discussed. The development of PC polymeric materials such as composites and blends are also addressed, emphasizing in particular the properties and the applications of impact modified PC blends and even of PC/Polyester systems.
47) Advances in Polymer based Materials and Related Technologies
C.Puglisi, S.Battiato, D.Carbone, A.Scamporrino, F.Samperi
Advances in Polymer based Materials and Related Technologies (Capri -NA May 29th - June 1st, 2011)
- 2011
The molar composition of the copolymers produced during the melt mixing of equimolar PC/PEN blend, in presence of Ti(OBu)4 (0.5%w) as a catalyst, was monitored as a function of the reaction time by 1H-NMR. 1H-NMR spectra show a series of new signals with respect to the initial homopolymers, indicating the formation of copolymers. They show also that the intensity of methylene (-CH2-)protons decreass with respect that of the aromatic protons of the naphthalate units, as increases the reaction time, indicating the elimination of ethylene carbonate (ETC), as well as in the PC/PET blend melt mixed in the similar conditions. The exchange reactions were monitored by 13C-NMR following the intensity changes of signals corresponding to the -CH2- (62-68 ppm) and to the BPA (114-121 ppm). Applying an appropriate statistical model and using the intensities of these signals we have calculated the molar fractions of each copolymer component. We have found that after 2 min reactive blending the copolymer is essentially constituted of the expected four-component. With the progress of the reaction which occurring, this become a five- components from 5 min up to 10 min, and then become a six component after 15 min up to 45 min. After this time the sequences containing carbonate units disappear owing the elimination of CO2 and ETC, and a four-component copolymer is formed. The elimination of carbonate units was also followed looking the carbon signals due to the corresponding carbonyl that resonate between 153 and 155 ppm and was also confirmed by FT-IR analysis. The weight loss of ETC and CO2 was calculated from NMR data, ando also by isothermal TGA analysis at 280 °C. The DSC data show that the TG of the copolymers changes as a function of their composition and we have proposed and equation to predict this characteristic.
48) Fluorene Based Copolymers For Solid-State Lighting: Correlation Between Molecular Structure And Electro-Optical Properties
U.Giovanella, M.Pasini, A.Bolognesi, F.Samperi, S.Battiato, S.Destri
XX Convegno Italiano di Scienza e Tecnologia delle Macromolecole (AIM) TERNI 4 - 8 Settembre 2011
- 2011
Efficient white electroluminescence can be obtained from a fully miscible blend of a polyfluorene-based copolymers emitting with the three fundamental colours and their chemical composition is a key factor to tune the emission properties. By combining physical and chemical characterization techniques it is possible to correlate copolymer molecular structures and electro-optical properties.
49) Synthesis and Characterization of Linear Hyperbranched PBA Block Copolymers
L.Ascione, V.Ambrogi, C.Carfagna, C.Puglisi, S.Battiato, F.Samperi
5th International conference on times of polymers (top) & composites (Ischia)
- 2010
50) Characterization of Copolyesteramides from Reactive Blending of PET and MXD6 in the Molten State
F.Samperi, M.S.Montaudo, S.Battiato, D.Carbone, C.Puglisi
Journal of Polymer Science: Part A: Polymer Chemistry
48,
5135-5155
- 2010
Poly(ethylene terephthalate)-poly(m-xylylene adipamide) PET-MXD6 copolymers were prepared by reactive blending of equimolar PET/MXD6 blends at 285 °C for different times in presence of terephthalic acid (1 wt %). First, the partial hydrolysis of PET and MXD6 occurs, yielding oligomers terminated with the reactive aromatic carboxyl groups. These oligomers quickly react with ester and amide inner groups producing a PET-MXD6 copolymer that may compatibilize the initial biphasic blend. In this homogeneous environment, the aliphatic carboxyl-terminated MXD6 chains, inactive in the initial biphasic blend, may promote the exchange reactions determining the formation of a random copolymer at longer reaction time (120 min). The progress of exchange reactions, and the microstructure of the formed copolyesteramides, versus the reaction time was followed by 1H and 13C NMR analyses using a CDCl3/TFA-d/(CF3CO)2O mixture as solvent and applying appropriate mathematical models. Dyads and triads sequences were thoroughly characterized by NMR. Semicrystalline block copolymers were obtained at reaction time lower than 45 min. All PET-MXD6 copolymers show a single Tg that change as a function of the dyads molar composition in the copolymers. The measured Tg values match with those calculated by a proposed modified Fox equation that take into account the weight fraction of the four dyad components of the PET-MXD6 copolymers.
51) Amino terminated copoly(ethersulphone)s bearing biphenylenic units in the backbone: Synthesis and characterization
A.Mamo, A.Aureliano, S.Battiato, G.Cicala, F.Samperi, A.Scamporrino, A.Recca
Polymer
51(14),
2972-2983
- 2010
The present project is based on the synthesis of new essentially amino-ended copoly(ether sulphone)s which are characterized by the presence of rigid biphenyl units in the main chain. All the compounds have been fully characterized by (1H and 13C) NMR, FTIR, and MALDI-TOF MS techniques. Complete assignments of the 1H and 13C NMR spectra have been accomplished by comparison with model compounds and literature data. The thermal properties of the copolymers were studied by differential scanning calorimetry (DSC). The glass transition temperature of the copolymers increases as increasing the molar composition of ethere sulphone units containing biphenyl moiety. The copolymers synthesized might find application as reactive toughening agents for multifunctional epoxy resins when high thermal properties are a requirement.
52) Synthesis and Characterization of Sulfonated Copolyethersulfones
F.Samperi, S.Battiato, C.Puglisi, V.Asarisi, A.Recca, G.Cicala, R.Mendichi
Journal of Polymer Science part A: Polymer Chemistry
48,
3010-3023
- 2010
The present article deals with the synthesis and characterization of some sulfonated copolyethersulfones. The synthetic approach differs from the post sulfonation approach traditionally reported in the literature. The synthetic procedure is based on the use of sulfonated monomers which are then reacted with previously synthesized telechelic hydoxy-ended poly (ether sulpnone)s. Combining the MALDI-TOF MS and 1H NMR analyses, with SEC-Viscometry and TGA measurements, we demonstrate a powerful tool for characterizing the chemical composition, end chains, degree of sulfonation (DS) and molecular mass distribution (MMD) of disulfonated poly(arylene ether-sulfone) copolymers. The characterization techniques allowed to determine the exact nature of the copolymers synthesized and to reveal some interesting features about the reaction. DMA data show that the glass transition temperature of sulfonated copolymers with similar DS increase as raise their MMD. Copolymers with a DS of 10-11 mol % reach a Tg of 244-246 °C.
53) Structure-Property Relationships in Densely Grafted ð-Stacked Polymers
A.Cappelli, M.Paolino, P.Anzini, G.Giuliani, S.Valenti, M.Aggravi, A.Donati, R.Mendichi, L.Zetta, A.C.Boccia, F.Bertini, F.Samperi, S.Battiato, E.Paccagnini, S.Vomero
Journal of Polymer Science part A: Polymer Chemistry
48(11),
2446-2461
- 2010
Three methyl end-capped oligo(ethylene glycol) (MOEG) ethers (1b-d) and a methoxyderivative (1a) of benzofulvene monomer BF3k were synthesized and induced to polymerize spontaneously by solvent removal to obtain soluble π-stacked polymers bearing densely grafted MOEG side chains (poly-1b-d) and model polymer poly-1a. The physicochemical features (e.g., solubility, NMR, MALDI-TOF, and absorption/emission spectra, as well as MWD, conformation plot, and thermal properties) of the synthesized polymers were compared in a structure-property relationship study. This approach afforded the following evidence. The structure of poly-1a-d is very similar to that of BF3k, suggesting that the polymerization mechanism is not affected by the presence of the electron-rich methoxy group or bulkier MOEG side chains. However, the latter appear to be capable of affecting the conformational behavior of the polymer backbone. The solubility of poly-1a-d depends on the number of the oligo (ethylene glycol) monomeric units. In particular, poly-1d, featuring a long MOEGside chain (n = 9), shows an amphiphilic character and is soluble in a number of organic solvents, whereas it interacts with water to give isolated macromolecules in equilibrium with nanosized water-soluble aggregates.
54) Synthesis and Characterization of disulfonated Poly(arylene ether sulphone) Copolymers
F.Samperi, G.Cicala, V.Asarisi, I.Blanco
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp.2.59-2.60 (MI 13-17 Settembre 2009)
- 2009
55) Frazionamento, Analisi e caratterizzazione di Sistemi macromolecolari Complessi
R.Mendichi, A.Giacometti Schieroni, D.Piovani, F.Samperi
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp.2.5-2.6 (MI 13-17 Settembre 2009)
- 2009
56) Synthesis and Characterization of Oligo-(ethylene glicol) Conjugated Benzofulvene Macromonomers
R.Mendichi, A.C.Boccia, F.Bertini, L.Zetta, F.Samperi, A.Cappelli
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp.1.32-1-33 (MI 13-17 Settembre 2009)
- 2009
57) Characterization of New Copolymers Formed by Reactive Blending of PC/PEN Blends in the Molten State
F.Samperi, S.Battiato, C.Puglisi
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp. 3.34-3-35 (MI 13-17 Settembre 2009)
- 2009
58) Synthesis of Block Copolymers as Compatibilizers in Nylon6/Polycarbonate Blends
C.Puglisi, F.Samperi, A.Turturro
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp.3.30 (MI 13-17 Settembre 2009)
- 2009
59) Caratterizzazione dei Copolimeri PET-MXD6 Ottenuti per Miscelazione Reattiva del PET con MXD6 allo Stato Fuso
S.Battiato, F.Samperi, C.Puglisi, D.Carbone
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp.3.1-3-2 (MI 13-17 Settembre 2009)
- 2009
60) Chemical Reactions occurring in the molten state between Ny6 and Oxazoline groups linked to a cyclophosphazene moiety
F.Samperi, S.Battiato, R.Scaffaro, L.Botta, M.C.Mistretta, R.Bertanti, R.Milani
Atti del XIX Convegno Italiano di Scienza e Tecnologia delle Macromolecole - AIM pp. 3.3-3-4 (MI 13-17 Settembre 2009)
- 2009
61) Characterization of new copolymers formed by reactive blending of pc and pen
F.Samperi, S.Battiato, C.Puglisi
Atti del Convegno "Polymerfest " pp. 251-252 ( PA 30 Agosto- 2 Settembre 2009)
- 2009
62) Characterization of pet-mxd6 copolymers formed by reactive blending of pet/mxd6 blends in the molten state
S.Battiato, F.Samperi, C.Puglisi, D.Carbone
Atti del Convegno "Polymerfest " pp. 100-101 ( PA 30 Agosto- 2 Settembre 2009)
- 2009
63) Chemical Reactions occurring in the molten state between Ny6 and Oxazoline groups linked to a cyclophosphazene moiety
F.Samperi, S.Battiato, R.Scaffaro, L.Botta, M.C.Mistretta, R.Bertanti, R.Milani
Atti del Convegno "Polymerfest " pp. 98-99 ( PA 30 Agosto- 2 Settembre 2009)
- 2009
64) Caratterizzazione di copolimeri ottenuto per miscelazione reattiva di PET e MXD6
M.S.Montaudo, F.Samperi, S.Battiato
Giornata Tecnologica "Materie Plastiche per il Packaging: Novità dal mondo della Ricerca" (MI 27 Marzo 2009)
- 2009
65) Synthesis and characterization of poly(4-oxyalkylenoxy benzoate)s of different chain length
V.Siracusa, N.Lotti, C.Puglisi, F.Samperi, A.Munari, L.Finelli, S.Battiato
E-Polymers
9,
761-775
- 2009
Three different monomers starting from p-(4-hydroxy)benzoic acid and 3-chloro-1-propanol, 4-chloro-1-butanol and 6-chloro-1-hexanol, respectively, were synthesized and extensively characterized by means of 1H and 13C NMR, MALDI-TOF MS and elemental analysis. Subsequently, poly(4-oxyalkylenoxy benzoate)s polymers with different chain length were prepared in bulk starting from the mentioned above monomers, employing Ti(OBu)4 as catalyst, according to the usual two-stage polymerization procedure. The polymers were characterized in terms of chemical structure, molecular mass and thermal behaviour. Tg, Tm and the ability to crystallize were found to be affected by the number of methylene groups per repeat unit and the results were explained mainly on the basis of the increment of the chain flexibility.
66) Reactions Occurring during the Melt Mixing of Nylon 6 and Oxazoline-
Cyclophosphazene Units
F.Samperi, S.Bazzano, S.Battiato, R.Scaffaro, L.Botta, M.C.Mistretta, R.Bertani, R.Milani
Macromolecules
42,
5579-5592
- 2009
Specific reactions of amino and carboxyl end groups of Nylon 6 with the reactive oxazoline groups belonging to a cyclophosphazene compound (referred as CP2OXA) were carried out at 240° C for different times, under inert atmosphere. Ny6 polymers terminated with one specific reactive chain end (-COOH or NH2) were reacted with different amounts of CP2OXA to study the kinetic order of the reactions. All Ny6-CP2OXA reacted products soluble in trifluoroethanol (TFE) were well characterized by MALDI-TOF MS, FT-IR and (1H and 13C) NMR techniques. The MALDI-TOF results show that the oxazoline rings react with the carboxyl chain ends of Ny6 following second-order kinetics. The reactions with amino chain ends are very fast and high amounts of gels are produced in just 5 min heating, whereas traces of gel are formed when Ny6COOH and CP-2OXA are reacted for 60 min. The gels were characterized by FTIR analyses, and also by MALDI-TOF MS after partial acid hydrolysis. MALDI-TOF mass spectra reveal also that oxazoline rings can react with -NHamide groups along the Ny6 chains, and with secondary amino groups formed by a condensation reaction involving the elimination of ammonia from two amino chain ends. These side reactions could be responsible of the formation of the gels. The polymerization of CP2OXA was also observed, and the oligomers formed could also react with Ny6 samples during the melt mixing.
67) Effect on Structural Relaxation of the Poly(methyl-methacrylate) Copolymers Chain Flexibility
S.Della Sciucca, G.Spagnoli, M.Penco, S.Battiato, F.Samperi, R.Mendichi
Journal of Polymer Science part B-Polymer Physics
47(6),
596-607
- 2009
The structural relaxation of poly(methyl-methaerylate) (PMMA)-based copolymers with different chain flexibility has been studied by DSC with the classical procedure of the isothermal and dynamical approach. Modified PMMA with different chain flexibility have been prepared by free radical polymerization in solution using a mixture of monomers containing 10 mol % of alkyl methacrylate (i.e., ethyl, buthyl, and hexyl methacrylate). The molecular characteristics of all the prepared copolymers have been performed by a multiangle laser light scattering (MALS) photometer on-line to a size exclusion chromatography (SEC) system (SEC-MALS) after and before the thermal treatments, NMR (H-1 and C-13) and MALDI-TOF mass spectrometry. A comparison of the apparent relaxation rate (R-H) was appraised from the enthalpy loss by annealing the different samples at the same level of undercooling (T-a = T-g - 18 degrees C). It was found an increase of R-H increasing the chain flexibility in the copolymers. Dynamical tests, performed at different cooling rates, have been used to estimate the apparent activation energy of the relaxation process.
68) Synthesis and Spontaneous Polymerization of Oligo(ethylene glycol)-Conjugated Benzofulvene Macromonomers.
A Polymer Brush Forming a Physical Hydrogel
A.Cappelli, S.Galeazzi, G.Giuliani, M.Anzini, M.Grassi, R.Lapasin, G.Grassi, R.Farra, B.Dapas, M.Aggravi, A.Donati,L.Zetta, A.C.Boccia, F.Bertini, F.Samperi, S.Vomero
Macromolecules
42(7),
2368-2378
- 2009
Two methyl end-capped oligo(ethylene glycol) esters (1a,b) of benzofulvene derivative BF1 were synthesized and induced to polymerize spontaneously by solvent removal to give poly-1a,b showing both NMR and absorption/emission spectra very similar to those of poly-BF1. Poly-1a,b showed relatively high molecular weight and the tendency to depolymerize to a different degree as a function of the temperature in the presence of solvents, while they exhibited appreciable stability in the absence of solvent. Poly-1b, bearing a longer oligo(ethylene glycol) side chain, featured an amphiphilic character and interacted with a number of organic solvents to produce transparent gel aggregates, and with water to give a quite compact physical gel. Rheological studies performed on the hydrogel suggested strong gel characteristics and the combination of rheology and NMR transverse relaxation measurements allowed the pore size distribution in the hydrogel to be defined. Finally, biological studies performed with poly-1b solutions showed neither cytotoxicity nor cell viability impairment suggesting potential biocompatibility features for this polymer. In conclusion, poly-1b can be considered a promising polymer for the preparation of hydrogels potentially useful in a range of biological and biotechnological applications such as drug delivery, molecular recognition, biosensing, protein and DNA separation, micro- and nanofluidics, as well as tissue engineering.
69) Aliphatic hydrocarbons in metasomatized gabbroic xenoliths from Hyblean diatremes (Sicily): Genesis in a serpentinite hydrothermal system
E.Ciliberto, C.Crisafulli, F.C.Manuella, F.Samperi, S.Scirè, V.Scribano, M.Viccaro, E.Viscuso
Chemical Geology
258,
258-268
- 2009
Many tholeiite gabbro xenoliths from the Hyblean tuff-breccia deposits (Sicily, southern Italy) present mineralogical and geochemical evidence for hydrothermal alteration at different temperatures and water/ rock ratios. In some cases, the primary mineral assemblage has been entirely replaced by Na-rich alkali feldspar, chlorite/smectite interlayers, zeolites, aegirine–augite, titanite, zircon etc. Hence the chemical composition of such metasomatic rocks displays larger amounts of volatiles, alkalis, Zr, Hf, U, Th and lower Ca, Mg, Fe with respect to the original gabbro. Five hydrothermally altered gabbroic xenoliths were selected for thermal decrepitation and bulk gas analyses by quadrupole mass spectrometry. All the samples analyzed display the same Electron Impact-Direct Pyrolysis Mass spectra (EI-DPMS). These show a series of peaks differing by 14 mass units due to loss of methylene groups (–CH2), by a fragmentation process typical of saturated aliphatic and aliphatic–aromatic hydrocarbons. In addition, Fourier Transform Infrared (FT-IR) spectra of the samples present several bands typical of vibration frequencies of aliphatic hydrocarbons. The high-molecular-weight hydrocarbons observed probably originated from Fischer–Tropsch-type (FT-t) synthesis in the high temperature section of a serpentinite-hosted hydrothermal system. This suggestion may lend support to the recent hypothesis regarding the original oceanic nature of the Hyblean lithospheric basement.
70) Structural and chemical characterization of melted PET/MXD6 blends
S.Battiato, F.Samperi, C.Puglisi
XVII Congresso Nazionale di Chimica Industriale, Energia, materiali e prodotti da tecnologie e processi eco-sostenibili, Genova, 30 Giugno - 3 Luglio 2008
PLO-C05
- 2008
Exchange reactions occurring in the melt mixing processes of poly(ethylene terephthalate) (PET) with poly(m-xylene adipamide) (MXD6) blends at 290°C, in presence of p-TolueneSulfonic Acid (p-TsOH) or Terephthalic Acid (TA) as promoter reaction have been investigated by viscometry and 13C NMR tools. Equimolar mixtures (with respect to the repeat units) of PET/MXD6 blends were reacted in a glass reactor for different times (from 0 up to 100 min) under stirring and N2 flow. The inherent viscosity decrease with the reaction time until 60 min indicating that the molar mass of the blend decrease. After this reaction time the viscosity shows an increase slightly anomalous. This behavior may be due to the increase of the hydrodinamic volume of the formed copoly-(esteramide) and or an increase of the molar mass of the blend components owing of the condensation reactions which can occur during the melt mixing. On the base of the exchange reactions mechanism a four components PETMXD6 copolymers are expected. Their molar composition in terms of the molar fraction of dyads and triads sequences in the formed PET-MXD6 copolymers were monitored by 13C NMR analyses. These reveal the formation of copoly-(esteramide) PET/MXD6 with different degree of randomness as a function of mixing time as well as of the type and % (w/w) of the promoter reaction. For the same conditions of time and % of promoter reaction the greater degree of randomness was obtained with TA respect to p-TsOH. Up to 60 min of reaction time with the 1% of TA a block copoly-(esteramide) whit degree of randomness of about 0.5 was formed, while the blends processed until 100 min give a statistical copolymer (degree of randomness of about 0.9). Hand the other hand, increasing the percentage of TA up to 5% (w/w), for 60 min mixing, a random copolymers with similar degree of randomness of the Blend at 1% TA at 100 min was formed, but with less molar mass as showed the inherent viscosity measurements.
71) Reactive melt mixing of PEN/PC blends
F.Samperi, C.Puglisi
XVII Congresso Nazionale di Chimica Industriale, Energia, materiali e prodotti da tecnologie e processi eco-sostenibili, Genova, 30 Giugno - 3 Luglio 2008
PLO-C05
- 2008
Exchange reactions occurring in the melt mixing processes of poly(ethylene naphthalate) (PEN) with poly(Bisphenol-A carbonate) (PC) blends at 280°C in presence of Ti(OBu)4 as a catalyst, and under N2 flow, have been investigated by DSC and NMR (1H and 13C) tools. Equimolar mixtures (with respect to the repeat units) of PC/PEN blends were reacted in a mixer brabender for different times (from 0 up to 60 min). DSC analysis showed that products formed at 2 and 5 min heating present two glass transition (Tg) with temperature values intermediate to those of the homopolymers; materials formed at reaction time higher that 5 min show only one Tg value that increase as raise the mixing time. The molar composition, the molar fraction of dyads and triads sequences of PCPEN formed copolymers were monitored by 13C and 1H-NMR analyses. Besides the four component expected on the base of the exchange reactions stoichiometry, were also revealed the ether groups along the copolymer chains. These can be formed by elimination of CO2 from aliphatic carbonate units formed in the exchange reactions between PEN and PC. These aliphatic carbonate units can be also eliminate ethylene carbonate (ETC) generating a thermally stable aromatic ester units. NMR analyses showed that a random aromatic copolyester-ether with a very low amount of PEN (2% mol) units was formed at mixing time higher than 30 min. The weight loss of ETC and CO2, was monitored by NMR analyses and also by isothermal thermogravimetric analysis at 280°C of a PEN/PC sample in the presence of catalyst. Selective extraction of the melt mixed materials with specific solvents showed that 45%w and 90%w of PC-PEN copolymer was formed at 2 and 5 min of mixing, respectively.
72) Synthesis and characterization of novel polyamides from new aromatic phosphonate diamine monomer
P.Finocchiaro, G.A.Consiglio, A.Imbrogiano, S.Failla, F.Samperi, S.Bazzano, C.Puglisi, G.Scaltro
European Polymer Journal
44,
2639-2651
- 2008
New aliphatic-aromatic and fully aromatic phosphonate polyamides were prepared by polycondensation reaction of our synthesized aromatic diamine: tetraethyl[(2,5-diamino-3,6-dimethylbenzene-1,4-diyl)dimethanediyl]bis(phosphonate) with the specific di-acylchloride (adipoyl chloride, isophthaloyl chloride and terephthaloyl chloride). The chemical structure of all samples were characterized by (1H and 31P) NMR, MALDI-TOF MS, FT-IR tools, whereas their thermal properties were determined by DSC and TGA techniques. The phosponate polyadipamide (referred as PAP) is a semi-crystalline sample with a melting temperature at about 261° C and glass transition (Tg) of 71° C. All polymers show two thermal degradation steps in the temperature range 270-550° C. Each polymer, independently its structure, shows the first maximum rate of thermal decomposition temperature (PDT) around 300-310° C, which may be due to thermal degradation of phoshonate groups. MALDI-TOF spectra, beside the linear oligomers terminated with the specific groups expected in accord to the synthesis procedure, reveals the presence of cyclic oligomers in the polyadipamide and polyisophthalamide samples.
73) Stimuli-responsive poly(ampholyte)s containing L-histidine residues: synthesis and protonation thermodynamics of methacrylic polymers in the free and in the cross-linked gel forms
M.Casolaro, Y.Ito, T.Ishii, S.Bottari, F.Samperi, R.Mendichi
Express Polymer Letters
2,
165
- 2008
Abstract. Methacrylate-structured poly(ampholyte)s were synthesized in the homopolymer and copolymer forms starting from the N-methacryloyl-L-histidine (MHist) and the N-isopropylacrylamide (NIPAAm). They were also obtained in the cross-linked (hydrogel) form, showing a close thermodynamic behaviour as that shown by the corresponding soluble free polymer analogues. Viscometric data revealed that the minimum hydrodynamic volume of the polymer at its isoelectric point (pH 5) shifted to lower pHs as the NIPAAm content increased, and beyond a critical low MHist content the reduced viscosity decreased, even at low pHs. The phenomenon was attributed to hydrophobic forces between the isopropyl groups outweighing the repulsive electrostatic interactions of the polymer in the positively charged form. A similar behaviour was shown by the corresponding hydrogel. The latter also revealed a different phase transition phenomenon induced by external stimuli (temperature, pH, ionic strength, electric current) when compared to the acrylate-structured analogues. The polyMHist, as well as the corresponding monomer, was found for two days to be non toxic against the mouse osteoblasts (MC3T3-E1).
74) Thermal decomposition products of copoly(arylene ether sulfone)s characterized by direct pyrolysis mass spectrometry
F.Samperi, C.Puglisi, T.Ferreri, R.Messina, G. Cicala, A.Recca, C.L.Restuccia, A.Scamporrino
Polymer Degradation and Stability
92(7),
1304-1315
- 2007
Pyrolysis products with mass of up to 850 Da were detected by direct pyrolysis mass spectrometric (DPMS) analysis of a series of copoly(arylene ether sulfone)s (PESePPO) synthesized by nucleophilic condensation of either 4,4’-dichlorodiphenylsulfone (CDPS) or 4,4’-bis-(4-chlorophenyl sulfonyl) biphenyl (long chain dichloride, LCDC) with different molar ratios of hydroquinone (HQ) or dihydroxydiphenylsulfone (HDPS). Pyrolysis products retaining the repeating units of the initial copolymers were formed at temperatures ranging from 420º C to 470º C (near the initial decomposition temperature). At temperatures higher than 450º C were observed products containing biphenyl units, formed by the elimination process of SO2 from diphenyl sulfone bridges. Products having biphenyl and dibenzofuran moieties were detected in the mass spectra recorded at temperatures above 550º C. These units were formed by loss of hydrogen atom from diphenyl ether bridges. Although the EI (18 eV) mass spectra of the pyrolysis products of the samples investigated were very similar, it was found that the relative intensity of some ions reflects the molar composition of the copolymers analysed. Cyclic and linear oligomers with very low molecular mass, present in the crude copolymers, were also detected by DPMS. Thermogravimetric analysis also showed their excellent thermal stability below 400º C. It indicates that the copolymers yield a char residue of 40–45% at 800º C, which increases with the PPO mole fraction in the samples.
75) Synthesis and characterization of novel polyamides from new aromatic phosphonate diamine monomer
S.Bazzano, F.Samperi, C.Puglisi, P.Finocchiaro, G.A.Consiglio, A.Imbrogiano, S.Failla.
Atti del XVIII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Catania 16-20 Settembre 2007
,
211
- 2007
76) Pirolisi di materiali da imballaggio in tetrapack per la preparazione di carboni attivi
F.Samperi, T.Ferreri, C.Puglisi, D.Zampino, A.Bonaccorso, C.Crisafull
Atti del XVIII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Catania 16-20 Settembre 2007
,
211
- 2007
77) Effect on structural relaxation of the pmma copolymers chain flexibility
S.Della Sciucca, G.Spagnoli, M.Penco, L.Sartore, F.Samperi, S.D’Antone
Atti del XVIII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Catania 16-20 Settembre 2007
,
211
- 2007
78) Mechanism of Reactions Occurring in PEN/PC Blends During The Melt
C.Puglisi F.Samperi
Mixing Process MoDeSt 2007. Workshop on DEGRADATION AND STABILIZATION OF POLYMER BLENDS Palermo, 9 September 2007
- 2007
79) Characterization of Nylons -Based Copolymer Prepared by Reactive Melt-Mixing
C.Puglisi, F.Samperi
Atti del 9th EUROPEAN SYMPOSIUM Polymer Blends, September 9-12, 2007, Palermo, Italy
,
18
- 2007
80) Exchange Mechanisms in the Reactive Melt-Mixing of Polyester and Polyamide Blends
F.Samperi, C.Puglisi
Atti del 9th EUROPEAN SYMPOSIUM Polymer Blends, September 9-12, 2007, Palermo, Italy
- 2007
81) Compatibilization of polymer blends by using a ciclophosphazene additive
R.Scaffaro, L.Botta, M.C.Mistretta, F.Samperi, S.Bazzano, C.Puglisi
Atti del 9th EUROPEAN SYMPOSIUM Polymer Blends, September 9-12, 2007, Palermo, Italy
,
17
- 2007
82) Reactivity of cyclophosphazenes with end functionalised Ny6 samples and EEA Copolymer
F.Samperi, C.Puglisi, S.Bazzano, R.Scaffaro, L.Botta, M.C.Mistretta
Atti del 9th EUROPEAN SYMPOSIUM Polymer Blends, September 9-12, 2007, Palermo, Italy
,
16
- 2007
83) Full Characterization of a Multiblock Copolymer Based on Poly(2,6-dimethyl-1,4-phenylene oxide) and Poly(bisphenol-A
carbonate)
F.Samperi, R.Mendichi, L.Sartore, M.Penco, C.Puglisi
Macromolecules
39(26),
9223-9233
- 2006
Full characterization, that is the true molar mass distribution and block sequence, of a multiblock copolymer based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and poly(bisphenol A carbonate) (PC) segments (PPO-b-PC), synthesized by polycondensation reaction of both diol-terminated PPO and PC samples with the bischloroformate of bisphenol A, is reported. The initial diol terminated PPO sample contains a few tetramethyl bisphenol A units in the backbone. The molar mass distribution of the starting PPO and PC homopolymers and the multiblock structure of the final PPO-b-PC copolymers were studied by light scattering and viscometry online with a SEC system, MALDI-TOF mass spectrometry, and NMR. In a previous study, we have demonstrated that homogeneous PPO-b-PC block copolymers having only one single Tg were obtained if low molar mass PPO and PC starting blocks were used. More important findings of this recent study are the following. MALDI-TOF analysis showed that the synthesized PPO-b-PC copolymers were composed of multiblock PPO-b-PC chains and also of some nonreacted PC oligomers terminated with methyl carbonate groups. Exhaustive and selective aminolysis of carbonate groups of the PC blocks has been also performed in order to determine the number of blocks and the average length of PPO blocks in the copolymers.
84) On the Preparation and Characterization of Polyethylene/Polyamide Blends by Melt Processing in the Presence of an Ethylene/Acrylic Acid Copolymer and of New Phosphazene Compounds
R.Scaffaro, M.C.Mistretta, F.P.La Mantia, M.Gleria, R.Bertani, F.Samperi, C.Puglisi
Macromolecular Chemistry and Physics
207,
1986-1997
- 2006
Samples of HDPE and PA6 have been meltprocessed in the presence of two new phosphazene compounds, CP-2EPOX and CP-2OXA together with an ethylene/acrylic acid copolymer. The blends have been prepared in an industrial twin-screw extruder by using PA6 and PE in weight ratios of 25/75 and 75/25. When used, 5 phr of EAA and 0.2 phr of CP have been added. The materials have been completely characterized from a rheological, morphological, and mechanical point of view. The results indicate that the additives used caused an increase in the rupture tensile properties, of the impact strength and viscosity especially in the PE-rich blend in the presence of CP-2EPOX. This result can be attributed both to a chain extension reaction on EAA and PA6 phases and on compatibilization effect due to the possible formation of EAA-g-PA6 copolymers. This latter occurrence is suggested by an improved adhesion between the phases and by an increased turbidity observed in the Molau tests. Between the two compatibilizers, the CP-2EPOX displays the overall best results, especially for the PE-based blends.
85) Novel Therapeutic Agents for Bone Resorption. Part 1. Synthesis and Protonation Thermodynamics of Poly(amido-amine)s Containing Bis-phosphonate Residues
M.Casolaro, I.Casolaro, A.Spreafico, C.Capperucci, B.Frediani, R.Marcolongo, N.Margiotta, R.Ostuni, R.Mendichi, F.Samperi, T.Ishii, Y.Ito
Biomacromolecules
7(12),
3417-3427
- 2006
Two poly(amido-amine)s (oligoPAM and oligoNER) contg. bis-phosphonate residues were obtained by a Michael-type polyaddn. of pamidronate and neridronate to 1,4-bis(acryloyl)piperazine. The SEC (size-exclusion chromatog.) and the MALDI-TOF (matrix assisted laser desorption ionization) analyses were consistent with the presence of oligomeric species (2-3 kDa) and with a narrow polydispersity index. The thermodn. results (log Ks, -Δ H° , and Δ S° obtained at 25°C in 0.15 M NaCl) of both the oligomers and the corresponding low mol. wt. precursors were in line with a cluster structure formed during the protonation of the basic nitrogen in the pamidronate. The soly. of the oligoNER with a longer aliph. chain was improved at high pHs, allowing the evaluation of their soln. properties. Preliminary biol. results show that both the oligomers do not neg. affect the in vitro viability, proliferation, and cellular activity of either normal animal or human osteoblasts.
86) Preparation of Block Copolymers with a Single Tg Based on Segments of Poly(oxy-2,6-dimethyl-1, 4-phenylene) and Polycarbonate of Bisphenol A
M.Penco, L.Sartore, S.Della Sciucca, L.Di Landro, R.Mendichi, F.Samperi
Macromolecular Chemistry and Physics
207,
1492-1500
- 2006
The synthesis and the properties of block copolymers based on PPO and PC segments are reported. Copolymers that have a multi-block structure are synthesized by a polycondensation reaction that employs oligomeric PPO and PC diol terminated with phosgene or bischloroformate of bisphenol A. In the reaction scheme two steps are involved: first, the reaction of one of the oligomeric diols (PPO or PC) with the bischloroformate or phosgene; second, the oligomeric bischloroformate is reacted with the other diol. The molecular characteristics of the prepared samples are studied by SEC, 1H and 13C NMR, and FT-IR spectroscopy. The thermal and rheological properties and the thermal stability have also been investigated bymeans ofDSC, rotational rheometry, andTGA, respectively. Polymers that have a single glass transition temperature are obtained if low-molecular-weight segments are used. From a rheological point of view, thesematerials show a remarkably lower melt viscosity compared with a PPO homopolymer that has a comparable average molecular weight.
87) Effect of adding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends. II: Effect of different polyamides on the properties of extruded samples
R.Scaffaro, L.Botta, F.P.La Mantia, M.Gleria, R.Bertani, F.Samperi, G.Scaltro
Polymer Degradation and Stability
91(10),
2265-2274
- 2006
Poly(butylene terephthalate) (PBT) and a sample of polyamide have been melt processed in the presence of two new phosphazene compounds, namely 2,2-dichloro-4,4,6,6-bis[spyro(2′,2"-dioxy-1′,1"-biphenyl)]cyclotriphosphazene (2Cl-CP) and 2,2-bis(2-methoxy-4-methyleneoxy-phenoxy)-4,4,6,6-bis[spyro(2′,2"-dioxy-1′,1"-biphenyl)]cyclophosphazene (CP-2EPOX). The blends were prepared by using polyamide 6 (PA6) and polyamide 6,6 (PA66) in 25/75 and 75/25 w/w compositions by using a co-rotating twin-screw extruder. The materials have been completely characterized from a mechanical, rheological, and morphological point of view. The results indicate that the additives used cause an increase of the rupture properties and of the viscosity, especially in the PA6 rich blends containing CP-2EPOX. This result can be not only attributed to a chain extension effect on the PA phase but also to in situ formation of PA/PBT copolymers promoted by the presence of the CP compound as confirmed by NMR and MALDI-TOF analyses. The compatibilization effect fades in blends containing PA66, probably due to a thermal deactivation of the additives at higher temperature required to process this polymer. The materials have been completely characterized from a mechanical, rheological, and morphological point of view. The results indicate that the additives used cause an increase of the rupture properties and of the viscosity, especially in the PA6 rich blends containing CP-2EPOX. This result can be not only attributed to a chain extension effect on the PA phase but also to in situ formation of PA/PBT copolymers promoted by the presence of the CP compound as confirmed by NMR and MALDI-TOF analyses. The compatibilization effect fades in blends containing PA66, probably due to a thermal deactivation of the additives at higher temperature required to process this polymer.
88) Combined MALDI-TOF MS and NMR characterization of copoly(arylen ether sulphone)s
C.Puglisi, F.Samperi, G.Cicala, A.Recca, C.L.Restuccia
Polymer
47(6),
1861-1874
- 2006
The synthesis and the chem. characterization of some novel random copolyethersulfones of the type P(ESES-co-EES) (Polyethersulfone-ethersulfone:Polyetherethersulfone), is reported. The ESES:EES molar ratio was varied from 80:20 to 20:80 and all the copolymers were terminated with reactive amino groups, and then were fully characterized through (1H and 13C)-NMR and MALDI-TOF MS anal. to confirm their chem. structures and chem. compns. End chains were revealed by these techniques. Contrary to chlorine-ended copolymers, oligomers terminated with hydroxyl groups were revealed in the amine-ended copolymers, indicating that transetherification reactions occur during the capping reaction with m-aminophenol. Cycles were also revealed by MALDI-TOF MS, that revealed also some unexpected end chains may be formed for the impurities in the feed. The glass transition temps. of the copolymers have been detd. by differential scanning calorimetry (DSC).
89) Characterization of synthetic polymers by MALDI-MS
G.Montaudo, F.Samperi, M.S.Montaudo
Progress in Polymer Science
31,
277-357
- 2006
In recent years, matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy has become a routine analytical tool for the structural analysis of polymers, complementing NMR and other traditional techniques, a noteworthy change with respect to the past, when mass spectrometry (MS) was seldom used. In this review, we discuss salient aspects of MALDI. First, we devote a section to fundamentals and practice in MALDI of polymers (such as the laser, ion source, ion optics, reflectron, detector, ionization efficiency) as well as to some basic concepts of sample preparation (such as the MALDI matrix and cationization agents). Then, we focus on measurable quantities of polymers: average molar masses, the chemical formula and the structure of the monomer (actually of the repeat unit), the masses of the chain end groups, etc. In-depth coverage is given of coupling MALDI with liquid chromatography (LC), since often LC offers valuable help in exploring macromolecules. The final section is devoted to recent applications, with a detailed discussion of MALDI of addition polymers, condensation polymers, polymers with heteroatoms in the chain, copolymers and partially degraded polymers.
90) Sintesi e caratterizzazione di copoliammidi NY6/NY6,10 e NY6/NY4,6
C.Puglisi, F.Samperi, G.Montaudo
Atti del XVII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Napoli 11-15 settembre 2005
,
81
- 2005
91) Sintesi e caratterizzazione di polieterisolfoni PES-PEES
G.Cicala, G.Magnano, A.Recca, C.L.Restuccia, F.Samperi, C.Puglisi.
Atti del XVII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Napoli 11-15 settembre 2005
,
77
- 2005
92) Caratterizzazione strutturale di copolimeri ottenuti mediante miscelazione reattiva controllata
F.Samperi, C.Puglisi, G.Montaudo
XVII Convegno Italiano di Scienza e Tecnologia delle Macromolecole, 11-15 settembre 2005, Napoli
,
73
- 2005
93) Current trends in matrix-assisted laser desorption/ionization of polymeric materials
G.Montaudo, F.Samperi, M.S.Montaudo, S.Carroccio, C.Puglisi
European Journal of Mass Spectrometry
11(1),
1
- 2005
In the last few years, mass spectrometry has rapidly become indispensable in polymer analysis and complements in many ways the structural data provided by nuclear magnetic resonance. Mass spectrometry of polymers is emerging as a revolutionary technique, capable of challenging the techniques and protocols established for years for the characterization of synthetic polymers. Matrix-assisted laser desorption/ionization (MALDI) has become a widely applied method for the structural characterization of synthetic polymers. The primary aim of this review is to illustrate some recent advances in the study of macromolecular systems by MALDI. MALDI allows the identification of repeat units and end groups, the structural analysis of linear and cyclic oligomers and the estimate of composition and sequence for copolymers. MALDI is also quite useful for the measurement of molar mass and bivariate distributions in polymers and for the detection of self-association in macromolecules, performed by coupling MALDI and size exclusion chromatography (SEC). Recently MALDI has been applied with remarkable success to the study of thermal and oxidative processes in polymers and to the characterization of copolymers obtained by reactive polymer blending. Selected applications of MALDI to polymers are illustrated herewith.
94) Thermal degradation of poly(ethylene terephthalate) at the processing temperature
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Polymer Degradation and Stability
83(1),
3
- 2004
Several isothermal degrdn. expts. on poly(ethylene terephthalate) (PET) were conducted in the temp. range of 270-370°C in order to simulate reactions that take place during the processing of PET under a nitrogen atm. Structural characterization of the reaction products was performed by MALDI mass spectrometry and by NMR anal. The results indicate the formation of cyclic oligomers that decomp. at higher temp. Vinyl ester-terminated oligomers could not be detected by MALDI or by 1H and 13C NMR, whereas the formation of anhydride-contg. oligomers was noted. Formation of acetaldehyde in PET samples processed at various temps. was detected by 1H NMR. We have also included in the present study a set of expts. where 0.5 wt.% p-toluenesulfonic acid hydrate (I) was added to the polyester. Our results show that the addn. of small amts. of I and heating the compn. at 270 or 285°C induces a strong hydrolytic reaction with consequent increase of carboxy-terminated polyester chains.
95) Thermal degradation of poly(butylene terephthalate) at the processing temperature
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Polymer Degradation and Stability
83(1),
11
- 2004
We previously reported an investigation on the isothermal degrdn. of poly(ethylene terephthalate) (PET) and present here a parallel study on poly(butylene terephthalate) (PBT). Although the two polyesters are structurally quite similar, our results show that the presence of the butylene unit in PBT is apparently able to induce significant changes in the isothermal degrdn. of PBT compared to PET. A rationalization of the differences obsd. is offered. Several isothermal degrdn. expts. on PBT were conducted at 270-350°C in order to simulate the reactions that take place in its actual thermal processing of. Structural characterization of the reaction products was performed by MALDI mass spectrometry and by NMR anal. The results indicate that cyclic oligomers of PBT formed at temps. below 290°C by ring-chain equilibration undergo thermal decompn. at higher temps. by a β-hydrogen transfer mechanism, as well as the open PBT chains. The formation of unsatd. oligomers was detected by MALDI and also by 1H and 13C NMR techniques, whereas in contrast to the thermally degraded PET sample, terephthalic anhydride-contg. oligomers were not obsd. Formation of butadiene was suggested by 1H NMR data on PBT samples processed at various temps.
96) Exchange reactions occurring through active chain ends. MALDI-TOF characterization of copolymers from Nylon 6,6 and Nylon 6,10
C.Puglisi, F.Samperi, S.Di Giorgi, G.Montaudo
Atti del The 2nd International Conference on Times of Polymers (TOP) Ischia (Na) 20-23 giugno 2004
,
34
- 2004
97) Structural characterization of copolyamides synthesized via the facile blending of polyamides
F.Samperi, M.S.Montaudo, C.Puglisi, S.Di Giorgi, G.Montaudo
Atti del The 2nd International Conference on Times of Polymers (TOP) Ischia (Na) 20-23 giugno 2004
,
33
- 2004
98) Essential role of chain ends in the Nylon6/Poly(ethyleneterephthalate) exchange
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Atti del The 2nd International Conference on Times of Polymers (TOP) Ischia (Na) 20-23 giugno 2004
,
32
- 2004
99) Essential role of chain ends in the Ny6/ PBT exchange a combined NMR and MALDI approach
F.Samperi, Maurizio Montaudo, C.Puglisi, Rossana Alicata, Giorgio Montando
Atti del The 2nd International Conference on Times of Polymers (TOP) Ischia (Na) 20-23 giugno 2004
,
3
- 2004
100) Recent Advances in MALDI Mass Spectrometry or Polymers
G.Montaudo, S.Carroccio, M.S.Montaudo, C.Puglisi, F.Samperi
Macromolecular Symposia
218(1),
101
- 2004
Matrix-Assisted Laser Desorption/Ionization (MALDI) allows the identification of repeat units and end groups, the structural analysis of linear and cyclic oligomers, and the estimate of composition and sequence far copolymers. MALDI has also been applied to the measurement of molar mass distributions in polymers and to the study of thermal and oxidative processes in polymers. This paper illustrates the detection of self-association in macromolecules made by coupling MALDI and Size Exclusion Chromatography (SEC), the investigation of polymer oxidation phenomena, and the characterization of copolymers formed in the processing of reactive polymer blends.
101) Structural Characterization of Copolyamides Synthesized via the Facile Blending of Polyamides
F.Samperi, M.S.Montaudo, C.Puglisi, S.Di Giorgi, G.Montaudo
Macromolecules
37(17),
6449
- 2004
Ny6-Ny6,10 and Ny6-Ny4,6 copolyamides prepd. by a facile melt mixing at 290-310 °C of carboxyl terminated Nylon6 (Ny6-COOH) with Ny6,10 or Ny4,6, were characterized by MALDI, 13C NMR, and DSC anal. The results, from one side show how facile is the high yield synthesis of random copolyamides via the melt mixing of the corresponding polyamides. What makes the synthesis so facile is the use of a carboxyl terminated polyamide (Ny6-COOH) to attack the other polyamide (Ny4,6 or Ny6,10), as described herewith. The DSC data acquired provide a clear picture of the process. The second relevant point is about the sequence anal. of the copolyamides, made using their 13C NMR spectra. The sequence has been derived directly from chain statistics principles, avoiding the use of inadequate procedures. To our knowledge, this is the first time as far as condensation copolymers are concerned. Interesting, the sequence anal. of our copolyamides has been also performed independently, by extg. pertinent information from their MALDI spectra. The results have been found in excellent agreement with those from 13C NMR data.
102) Thermal degradation of PET and PBT at the processing temperature
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
26
- 2003
103) Caratterizzazione mediante MALDI-MS del gel formato per riscaldamento del Ny66.
F.Samperi, C.Puglisi, S.Di Giorgi, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
25
- 2003
104) Degradazione termica del policaprolattone
C.Puglisi, F.Samperi, N.Tasca, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
23
- 2003
105) Ruolo dei gruppi terminali nelle reazioni di scambio in miscele Ny6/PBT allo stato fuso
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
15
- 2003
106) Sintesi e caratterizzazione di copoliammidi Ny66/Ny6,10
F.Samperi, C.Puglisi, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
14
- 2003
107) Meccanismi di reazione in miscele di polimeri di condensazione allo stato fuso.
F.Samperi, C.Puglisi, G.Montaudo
Atti del XVI Convegno Italiano di Scienza e Tecnologia delle Macromolecole, Pisa 22-25 settembre 2003
,
10
- 2003
108) Exchange reactions occurring through active chain ends: MALDI-TOF characterization of copolymers from nylon 6,6 and nylon 6,10
C.Puglisi, F.Samperi, G.Montaudo
Abstracts of Papers, 225th ACS National Meeting, New Orleans, LA, United States, March 23-27, 2003
- 2003
A study on the sequence and compn. of copolyamides formed by activated exchange reactions occurring in the melt mixing of Ny6,10 and Ny6,6 is reported. Equimolar mixts. of Ny6,10, Ny6,6 and carboxyl terminated Ny6,6 samples were melt mixed at different temps. (290-310°C) for different times (10-180 min). MALDI-TOF mass spectra allowed the detection of the copolyamides formation and the detn. of their sequence and compn. The results can be reconciled within the overall scheme of exchange reaction occurring through active chain ends.The compn. of the copolyamides formed by the exchange of Ny6,6-COOH/Ny6,10 mixts. showed a higher amt. of Ny6,6 units, with respect to the initial blends compn., and a higher value of the Ny6,6 av. block length, at the beginning of the reaction (10 min). The compn. of the copolyamides was found to be equal to the feed compn. (50/50), after 30 min of heating, with a random distribution of sequences. The compn. and the sequence distributions of the copolyamides remained unaffected even at higher heating time (180 min) although some degrdn. reactions also occurred.
109) Essential role of chain ends in the nylon-6/poly(ethylene terephthalate) exchange
F.Samperi, C.Puglisi, R.Alicata, G.Montaudo
Journal of Polymer Science part A: Polymer Chemistry
41(18),
2778
- 2003
A combination of NMR and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) techniques were suitable tools for examg. the exchange reactions that occur during the melt-mixing of nylon-6 and poly(ethylene terephthalate) (Ny6/PET) blends in the presence of p-toluene sulfonic acid (TsOH) at 285 °C. Some researchers believe that TsOH is an efficient catalyst for the amide-ester exchange reactions in PET/Ny6 and PET/nylon-66 blends in the molten state. Instead, we have found that TsOH is able to react in the molten state with PET, yielding PET oligomers terminated with carboxyl groups. Because the latter oligomers can quickly react with Ny6 producing a Ny6/PET copolymer, the role of TsOH in the melt-mixing process is not that of a catalyst but of a reactant. Our study allowed the structural identification of the Ny6/PET copolyesteramide produced in the exchange as a function of melt-mixing time. The results revealed the essential role of carboxyl end groups in the exchange reaction between Ny6 and PET and allowed a detailed mechanism for this reaction.
110) Exchange reactions occurring through active chain ends. MALDI-TOF characterization of copolymers from nylon 6,6 and nylon 6,10
C.Puglisi, F.Samperi, G.Montaudo
Polymeric Materials Science and Engineering
88,
336
- 2003
A study on the sequence and compn. of copolyamides formed by activated exchange reactions occurring in the melt mixing of Nylon 6,10 and Nylon 6,6 is reported. Equimolar mixts. of Nylon 6,10, Nylon 6,6 and carboxyl terminated Nylon 6,6 samples were melt mixed at different temps. (290 - 310 °C) for different times (10 - 180 min). MALDI-TOF mass spectra allowed the detection of the copolyamides formation and the detn. of their sequence and compn. The results can be reconciled within the overall scheme of exchange reaction occurring through active chain ends. The compn. of the copolyamides formed by the exchange of Nylon 6,6-COOH/Nylon 6,10 mixts. showed a higher amt. of Nylon 6,6 units, with respect to the initial blends compn., and a higher value of the Nylon 6,6 av. block length, at the beginning of the reaction (10 min). The compn. of the copolyamides was equal to the feed compn. (50/50), after 30 min of heating, with a random distribution of sequences. The compn. and the sequence distributions of the copolyamides remained unaffected even at higher heating time (180 min) although some degrdn. reactions also occurred
111) Essential Role of Chain Ends in the Ny6/PBT Exchange. A Combined NMR and MALDI Approach
F.Samperi, M.S.Montaudo, C.Puglisi, R.Alicata, G.Montaudo
Macromolecules
36(19),
7143
- 2003
A combination of NMR and MALDI was found to be a suitable tool to study the exchange reactions that occur during the melt mixing of Nylon-6 and poly(butylene terephthalate) (Ny6/PBT) blends. The results reveal the essential role of carboxyl end groups in the exchange reaction, and allow drawing a detailed mechanism for this reaction. Using Ny6 and PBT samples bearing specific reactive end groups, it was demonstrated that only the carboxyl end groups of PBT and of Ny6 are able to react in the initially biphasic Ny6/PBT blends, so that an outer-inner exchange takes place (Scheme 3). The compn. of the copoly(esteramide) obtained at 260 °C in the exchange of Ny6/PBT shows a higher amt. of Ny6 units with respect to the initial blend compn. and a high value of the Ny6 av. sequence length. However, the compn. of the copoly(esteramide) obtained at 280 °C was instead found to be equal to the feed compn. (50/50), with a random distribution of av. sequence lengths. The results obtained in the present work can be reconciled within the overall model of exchange reaction occurring through active chain ends
112) Exchange Reactions Occurring through Active Chain Ends. MALDI-TOF Characterization of Copolymers from Nylon 6,6 and Nylon 6,10
C.Puglisi, F.Samperi, S.Di Giorgi, G.Montaudo
Macromolecules
36 (4),
1098
- 2003
A study on the sequence and composition of copolyamides formed by activated exchange reactions occurring in the melt mixing of Ny6,10 and Ny6,6 is reported. Equimolar mixtures of Ny6,10, Ny6,6, and carboxyl-terminated Ny6,6 (Ny6,6-COOH) samples were melt mixed at different temperatures (290-310 C) for different times (10-180 min). The determination of copolyamides formation, sequence, and composition proved to be unfeasible with 13C NMR spectroscopy. On the other hand, matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectra allowed the detection of the copolyamides formation and the determination of their sequence and composition. The results can be reconciled within the overall scheme of exchange reaction occurring through active chain ends. In fact, the composition of the copolyamides formed by the exchange of Ny6,6-COOH/Ny6,10 mixtures showed a higher amount of Ny6,6 units, with respect to the initial blends composition, and a higher value of the Ny6,6 average block length, at the beginning of the reaction (10 min). The composition of the copolyamides was found to be equal to the feed composition (50/50), after 30 min of heating, with a random distribution of sequences. The composition and the sequence distributions of the copolyamides remained unaffected even at a higher heating time (180 min) although some degradation reactions also occurred. Instead, the exchange reaction of a mixture of high molar mass Ny6,6 and Ny6,10 both containing a very low amount of carboxylic chain ends produced a segmented copolyamides even at high heating time (150 min). The Ny6,6/Ny6,10 copolyamides were also characterized by differential scanning calorimetry (DSC) and by wide-angle X-ray spectroscopy (WAXS)
113) Surface evolution of polycarbonate/polyethylene terephthalate blends induced by thermal treatments
A.Licciardello, A.Auditore, F.Samperi, C.Puglisi
Applied Surface Science
203,
556
- 2003
Bisphenol-A polycarbonate (PC) and polyethyleneterephthalate (PET) blends are known to undergo, upon thermal treatment (melt mixing), exchange reactions leading to the formation of copolymers having a final structure that is also affected by consecutive reactions involving CO2 and ethylene carbonate losses. In this work we followed the evolution of the surface composition of this system during the melt mixing at 270 degreesC, both with and without catalysts, by means of time-of-flight secondary ion mass spectroscopy (ToF-SIMS). The static SIMS spectra obtained at different treatment times show the appearance of peaks related to newly formed structures and also the modification of the relative intensities of peaks characteristic of both the initial constituents of the blend. From the variation of the relative intensities of peaks related to the bisphenol-A unit of PC and to the phthalate structure of PET, it is shown that after the first stages of melt mixing the surface is PC enriched and that with the progressive formation of a random copolymer the phthalate units increase their concentration at the surface of the system. Hence, as final result of the melt mixing process, the surface composition tends to reflect the relative amount of the repeating units in the bulk.
114) ToF-SIMS investigation of the thermally induced processes at the surface of polyester based polymer blends
A.Auditore, F.Samperi, C.Puglisi, A.Licciardello
Composites Science And Technology
63(8),
1213
- 2003
Surface evolution caused by thermal processing at 270 degreesC of blends of bisphenol-A polycarbonate (PC) with polyethyleneterephthalate (PET) or with polybuthyleneterephthalate (PBT) has been investigated by ToF-SIMS. Such systems are known, upon melt mixing, to undergo transesterification reactions leading to the formation of copolymers. In the case of PET-PC, the transesterification is accompanied by reactions involving the loss of small molecules from the resulting copolymer, whereas the copolymer resulting from the trans-esterification of PBT-PC is stable at the temperature under consideration. We report on Static SIMS measurements performed on blend samples melt mixed for different times, that show a complex evolution of surface composition, explained also in terms of surface-specific processes, such as segregation of lower surface free energy components. The results from the melt mixed system are compared with those obtained from parallel temperature-programmed ToF-SIMS experiment, performed on spin-cast thin films.
115) Thermal oxidation products of nylon-6 determined by MALDI-TOF mass spectrometry
G.Montaudo, C.Puglisi, F.Samperi, D.Chionna
Abstracts of Papers, 224th ACS National Meeting, Boston, MA, United States, August 18-22, 2002
- 2002
Thermal oxidative degrdn. products of Nylon 6 samples, generated at 250°C under atm. air were analyzed by Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) Mass Spectrometry. This technique possesses a high sensitivity, allowing the desorption and the ionisation of large mols., even in complex mixts., and therefore is widely used with increasing success for the characterization of synthetic polymers. The anal. of the MALDI-TOF spectra of thermally oxidized Nylon 6 samples showed the presence of polymer chains contg. aldehyde, amide,N-formamide, and Me end groups, which appear to be generated from primary oxidn. processes. The MALDI-TOF spectra show also the presence of carboxyl end groups, most likely formed by the further oxidn. of aldehyde contg. compds. Also the formation of azomethynes, from further reaction of aldehydes with amino terninated Ny6 chains, is also supported by the appearance of specific peaks in the MALDI spectra
116) MALDI-TOF characterisation of thermally generated gel from Nylon 66
C.Puglisi, F.Samperi, S.Di Giorgi, G.Montaudo
Polymer Degradation and Stability
78(2),
369
- 2002
Progress in understanding the thermal decomposition processes occurring in Nylon 66 (Ny66) was obtained by using matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry techniques. Ny66 samples were subjected to thermal degradation under inert atmosphere heating them at 290 and 315° C. The formation of a gel fraction was observed after about 15 min of heating and the addition of a condensing agent such as triphenyl phosphite (TPP) made the gelation complete in a few minutes. The MALDI-TOF mass spectra of the soluble fraction showed that secondary amino groups and cyclopentanone chain ends were generated in the heating process. The gel fraction was partially hydrolysed to destroy the network structure and the soluble material was analysed by MALDI-TOF. The spectra revealed the presence of N,N-substituted amide as side chains generated by the condensation of carboxyl end groups with secondary amino groups and azomethine structures originating from the reaction of cyclopentanone moieties with the terminaI amino groups. These structures were most likely responsible for the gel formation on heating Ny66 samples. Comparison with similar experiments conducted on Ny6, showed that only secondary amino groups were formed in Ny6, leading to branched structures but not to crosslinking.
117) End-Groups-Dependent MALDI Spectra of Polymer Mixtures
C.Puglisi, F.Samperi, R.Alicata, G.Montaudo
Macromolecules
35(8),
3000
- 2002
During a work designed to use MALDI-TOF MS to determine the composition of an equimolar blend of nylon 6 (Ny6) and polybutyleneterephthalate (PBT), the MALDI-TOF mass spectrum showed a surprising strong imbalance between the two components. The predominance of Ny6 oligomers over the PBT oligomers terminated with hydroxyl end chains is signaled by the appearance of specific peaks in the MALDI-TOF mass spectra. Since the average molar mass and the polydispersion of the two polymers are comparable, the MALDI-TOF MS ionization efficiency is quite different for the two components of the blend. This finding prompted us to a more detailed study, and to synthesize a number of Ny6 and PBT samples terminated with different end groups, to analyze their equimolar blends by MALDI-TOF MS. The results reported in the present study may help clarifying some fundamental aspects about the mechanisms of ions formation, when MALDI-TOF mass spectrometry is applied to macromolecules. End groups ionization efficiency appears to be the most important parameter in determining the relative intensity of peaks in the MALDI-TOF mass spectra of the polymer blends investigated. End-groups-dependent ionization of MALDI-TOF mass species is the key to rationalize the relative peak intensity in MALDI-TOF mass spectra of polymer mixtures. Understanding of ionization efficiency mechanisms is essential to the quantitative applications of MALDI-TOF MS.
118) Influence of chain end groups on the matrix-assisted laser desorption/ionization spectra of polymer blends
R.Alicata, G.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
16(4),
248
- 2002
The positive ion matrix-assisted laser desorption/ionization (MALDI) spectrum of an equimolar blend of Nylon 6 (Ny6) and hydroxyl-terminated polybutyleneterephthalate (PBT) shows a surprisingly strong imbalance between the two components. Since the average molar masses and the polydispersions of the two polymers are comparable, it follows that the efficiency of MALDI is quite different for the two components of the blend. This finding prompted us to a more detailed study, and to synthesize Ny6 and PBT samples terminated with different end groups, in order to analyze their blends by MALDI. The negative ion MALDI spectra of the mixtures investigated show that PBT samples do not yield signals, so that only Ny6 peaks appear in these spectra. By comparing positive and negative ion MALDI spectra of mixtures of Ny6 terminated with various end groups, it was found that the peak intensity depends on the nature of the end groups. The results reported in the present study may help to clarify some fundamental aspects of the mechanisms of ion formation, when MALDI mass spectrometry is applied to macromolecules. End group ionization efficiency appears to be the most important parameter in determining the relative intensity of peaks in the MALDI spectra of the polymer blends investigated. End-group-dependent ionization is the key to the rationalization of the relative peak intensities in MALDI spectra of polymer mixtures. Copyright (C) 2002 John Wiley Sons, Ltd.
119) Matrix-Assisted Laser Deserption Ionization Mass Spectrometry of Polymers (MALDI-MS)
G.Montaudo, M.S.Montaudo, F.Samperi
G.Montaudo, R.P.Lattimer (EDS) CRC Press
Chapter 10,
419
- 2001
120) Fast Atom Bombardment of Polymers
G.Montaudo, F.Samperi
G.Montaudo, R.P.Lattimer (EDS) CRC Press
Chapter 7,
269
- 2001
121) Thermal Oxidation Products of Nylon 6 Determined by MALDI-TOF Mass Spectrometry
D.Chionna, C.Puglisi, F.Samperi, G.Montaudo, A.Turturro
Macromolecular Rapid Communications
22(7),
524
- 2001
Matrix-assisted laser desorption ionisation (MALDI) mass spectrometry was used, in an attempt to find firm evidence for the structure of the species produced in the thermal oxidative degradation of Nylon 6 (Ny6), at 250 degreesC in air. The MALDI spectra of the products showed the presence of polymer chains containing aldehydes, amides, methyl and N-formamide terminal groups. The aldehydes undergo further oxidation to produce carboxylic end groups. The formation fo azomethines, from the further reaction of aldehydes with amino-terminated Ny6 chains, is also supported by the appearance of specific peaks in the MALDI spectra
122) Molar mass distributions and hydrodynamic interactions in random copolyesters investigated by SEC/MALDI
G.Montaudo, C.Puglisi, F.Samperi, M.S.Montaudo
Abstracts of Papers of the American Chemical Society
219,
432
- 2000
Results of d., ultrasonic velocity, and DSC measurements performed on aq. solns. of the homologous disaccharides trehalose, maltose and sucrose, are reported. To get some insight into the mechanisms of cryopreservation that characterize these systems, and to clarify the reasons that make trehalose the most effective bioprotector, we investigate the volumetric and thermic properties of trehalose, maltose and sucrose aq. solns. as a function of concn. and temp. What conclusively emerges is the presence of a more collapsed conformation for trehalose in respect with the other disaccharides, indicative of a much more marked solute-solvent interaction strength. Moreover, DSC findings indicate a greater effectiveness of trehalose in destroying the tetrahedral network of water compatible with the formation of ice and support the hypothesis of a higher "fragile" thermodn. character of the trehalose-water system at high diln.
123) MALDI-TOF investigation of polymer degradation: Pyrolysis of Poly(Bisphenol A carbonate)
G.Montaudo, C.Puglisi, F.Samperi, S.Carroccio
Abstracts of Papers of the American Chemical Society
219,
429
- 2000
124) SEC/MALDI analysis of poly(bisphenol a carbonate): Self-association due to end-groups
G.Montaudo, C.Puglisi, F.Samperi, S.Carroccio
Abstracts of Papers of the American Chemical Society
219,
241
- 2000
125) Copolymer Composition: a Key to the Mechanisms of Exchange in Reactive Polymer Blending
G.Montaudo, C.Puglisi, F.Samperi
S.Fakirov(Ed)WILEY-VCH.
Chapter 4,
159
- 1999
126) Maldi-Tof Investigation of Polymer Degradation. Pyrolysis of Poly(Bisphenol A Carbonate)
C.Puglisi, F.Samperi, S.Carroccio, G.Montaudo
Macromolecules
32(26),
8821
- 1999
The MALDI-TOF technique allows the detection of intact polymer molecules up to high masses and therefore offers the opportunity to study directly the thermal degradation processes occurring in polymeric materials by analyzing conveniently exposed (i.e., partially degraded) polymer samples. This opens new vistas in polymer analysis and deserves careful exploration, due to the relevance of polymer degradation phenomena in everyday practice. The thermal decomposition of poly(bisphenol A carbonate) (PC) has been investigated by heating isothermally at 300, 350, 400, and 450 degrees C and subsequent analysis of the pyrolysis residue by means of MALDI mass spectrometry. The MALDI mass spectra of the pyrolysis residues obtained at 300 degrees C showed a progressive reduction of the abundance of cyclic oligomers, whereas the relative abundance of the other compounds was unaffected. At 350 degrees C, the occurrence of an extensive hydrolysis reaction was responsible for the degradation of cycles and linear chains bearing tert-butylphenyl carbonate end groups with subsequent formation of abundant open chain PC oligomers with phenol end groups. Furthermore, at these two temperatures, cyclic oligomers never disappear in the MALDI spectra, even at higher heating time (60 min), suggesting the presence of an equilibrium between the rate of cleavage and the rate of formation of cycles. PC chains terminated with phenol groups at both ends, together with pyrolyzed chains bearing phenyl and isopropylidene end groups, generated by the disproportionation of the aliphatic bridge of bisphenol A, were observed in the MALDI spectra of pyrolysis residue obtained at 400 degrees C. Condensed aromatic compounds such as xanthones, which are considered to be the precursors of a graphite-like structure of the charred residue, were also detected in the MALDI spectra of PC samples heated at 400 degrees C and they became the most intense species at 450 degrees C. The PC samples heated at temperature higher than 450 degrees C consisted of insoluble carbonaceous materials not suitable for MALDI analysis
127) Analysis of Poly(Bispheno A Carbonate) by Size Exclusion Chromatography/Matrix-assisted Laser Desorption/Ionization.1.End Group and Molar Mass Determination
C.Puglisi, F.Samperi, S.Carroccio, G.Montaudo
Rapid Communications in Mass Spectrometry
13(22),
2260
- 1999
The determination of molar mass (MM) data for polydisperse polymers by SEC/MALDI involves the fractionation of samples through analytical size exclusion chromatography (SEC). Selected SEC fractions are then analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) and the mass spectra of these nearly monodisperse samples allow the determination of the average molar masses, The SEC/MALDI procedure has now been applied to two polycarbonate samples, PC1 and PC2, The results show that the MALDI spectra of the SEC fractions allow not only the detection of linear and cyclic oligomers contained in these samples, but also the simultaneous determination of their average molar masses. Two slightly differing SEC calibration plots were obtained, due to the smaller hydrodynamic volume of the polycarbonate cyclic chains with respect to the linear ones, In agreement with theory, the ratio (M-cycle/M-linear)v(e) at a fixed elution volume was found to be 1.22, independent of the molar mass values, Copyright © 1999 John Wiley & Sons, Ltd
128) Analysis of Poly(Bispheno A Carbonate) by Size Exclusion Chromatography/Matrix-assisted Laser Desorption/Ionization.2.Self-association Due to Phenol End Groups
C.Puglisi, F.Samperi, S.Carroccio, G.Montaudo
Rapid Communications in Mass Spectrometry
13(22),
2268
- 1999
We report here a case of apparent failure of the size exclusion chromatography/matrix-assisted laser desorption/ionization (SEC/MALDI) method to provide polymer fractions with narrow molar mass distribution, showing that intermolecular chain association is responsible for this phenomenon, Poly(bisphenol A carbonate) (PC) chains terminated with hydroxyl groups undergo self-association by hydrogen bonding, providing macromolecular aggregates with higher hydrodynamic volume. These aggregates are eluted through SEC columns at the same volume as higher molar mass chains, which remain non-associated. Thus, self-association affects negatively the SEC fractionation experiments, and even the sharpest SEC fractions contain a heterogeneous mixture of PC chains of different size. When the off-line SEC/MALDI procedure is applied, the SEC fraction is diluted in the matrix which, being a dissociating medium (carboxylic acid) for hydrogen-bonded aggregates, suppresses the chains' self-association. Therefore, the MALDI spectra of these PC fractions indicate a polydisperse character, with irregular bimodal distributions of peaks, As a consequence, in the presence of chain association, the SEC/MALDI method for the calculation of molar masses of polymers cannot be directly applied. In the present case we have found that, under opportune experimental conditions, self-association in polycarbonates can be avoided, so that nearly monodisperse SEC fractions can be obtained and the SEC/MALDI method can be applied, Our results also show that MALDI is a very sensitive technique for the detection of association of polymers in dilute solutions. Copyright © 1999 John Whey & Sons, Ltd
129) Mechanism of Exchange in BPT/PC and PET/PC Blends.Composition of the Copolymer Formed in the Melt Mixing Process
G.Montaudo, C.Puglisi, F.Samperi
Macromolecules
31(3),
650
- 1998
Mechanisms operating in the exchange reactions occurring in the melt mixing processes of Bisphenol A polycarbonate (PC) with poly(butylene terephthalate) (PBT) and poly(ethylene terephthalate) (PET) blends have been investigated making use of appropriate polymer samples, capped or containing reactive chain end groups. The exchange process may proceed by two different mechanisms: a direct exchange reaction between inner functional groups located inside the polymer chains, i.e., inner-inner, or by attack of reactive chain ends functional groups (outer) on inner groups, i.e., outer-inner. It is shown that the distinction between the two processes can be conveniently made by determining the composition of the copolymer formed in the exchange reaction. The inner-inner mechanism occurs only in the reaction between end-capped or high molar mass PET/PC or PET/PC samples, and it was found that the molar composition of the copolymer formed is always equal to the feed ratio of the two homopolymers and independent from the reaction time. The outer-inner mechanism occurs in the presence of hydroxyl or carboxyl reactive chain ends in PBT and PET samples. The reaction proceeds by the attack of the reactive end groups on the PC chains, originating block copolymers of PC and PBT and low molar mass PC with phenol end groups which are unreactive. The reaction stops right after the reactive end groups are consumed. The amount and the composition of the copolymers generated in the reactions are found to be constant as a function of time. The copolymer composition shows an excess of PBT or PET units with respect to the feed molar ratio. These results indicate that monitoring the composition of the copolymer formed in each case is diagnostic for establishing the mechanism of the reaction. The approach used here allows control of the composition and yield of the copolymer to be produced, and it is applicable to other systems where exchange reactions occur. [References: 32]
130) Molar Mass Distributions and Hydrodynamic Interactions in Random Copolyesters investigated by Size Exclusion Chromatography / Matrix Assisted Laser Desorption Ionization
M.S.Montaudo, C.Puglisi, F.Samperi, G.Montaudo
Macromolecules
31(12),
3839
- 1998
Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) was used in combination with size exclusion chromatography (SEC) to characterize a series of copolyesters with butylene adipate (BA), butylene succinate (BSu), and butylene sebacate (BSe) units. These copolyesters, synthesized by condensation polymerization from 1,4-butanediol and dimethyl esters, have flexible chains, are random, and do possess a homogeneous composition. The SEC fractionation of these polymers allowed to collect numerous fractions that were then analyzed by MALDI-TOF. The mass spectra of these nearly monodisperse fractions allowed the computation of the corresponding molar masses (MM). The calibrated SEC traces could then be used to compute average MM and molar mass distribution of the unfractionated samples. Furthermore, the SEC/MALDI data allowed an estimate of the effect of the hydrodynamic interactions of comonomers A and B along the copolymer chain and a comparison with the theoretical predictions. Sensible deviations from the simple additivity have been found for the four copolymers studied. [References: 17]
131) Chemical Reactions Occurring in the Thermal Treatment of PC/PMMA Blends
G.Montaudo, C.Puglisi, F.Samperi
Journal of Polymer Science part A: Polymer Chemistry
36,
1873
- 1998
The chemical reactions occurring in the thermal treatment of bisphenol-A polycarbonate( PC) and poly(methyl methacrylate) (PMMA) blends have been investigated by nuclear magnetic resonance (NMR), mass spectrometry (MS), size exclusion chromatography (SEC), and thermogravimetry (TG). Our results suggest that in the melt-mixing of PC/PMMA blends, at 230 degrees C, no exchange reactions occur and that only the depolymerization reaction of PMMA has been observed. In the presence of an ester-exchange catalyst (SnOBu2), an exchange reaction was found to occur at 230 degrees C, but no trace of PC/PMMA graft copolymer has been observed. Instead, an exchange reaction between the monomer methyl methacrylate (MMA), generated in the unzipping of PMMA chains, and the carbonate groups of PC has been suggested. This is due to the diffusion of MMA.at the interface or even into the PC domains, where it can react with PC producing law molar mass PC oligomers bearing methacrylate and methyl carbonate chain ends and leaving the undecomposed PMMA chains unaffected. The TG curves of PC/PMMA blends prepared by mechanical mixing and by casting from THF show two separated degradation steps corresponding to that of homopolymers. This behavior is different from that of a transparent film of PC/PMMA blend, obtained by solvent casting from DCB/CHCl3, which shows a single degradation step indicating that the degradation rate of PC is increased by the presence of PMMA in the blend. The thermal degradation products obtained by DPMS of this blend consist of methyl methacrylate (MMA), cyclic carbonates arising from the degradation of PMMA and PC, respectively, and a series of open chain bisphenol-A carbonate oligomers with methacrylate and methyl carbonate terminal groups. The presence of the latter compounds suggests a thermally activated exchange reaction occurring above 300 degrees C between MMA and PC. The presence of bisphenol-A carbonate oligomers bearing methyl ether end groups, generated by a thermally activated decarboxylation of the methyl carbonate end groups of PC, has also been observed among the pyrolysis products. © 1998 John Wiley & Sons, Inc. [References: 31]
132) Application of Size Exclusion Chromatography Matrix - assisted Laser Desorption/Ionization Time-of-flight to the Determination of Molecular Masses in Polydisperse Polymers
M.S.Montaudo, G.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
12(9),
519
- 1998
The determination of molecular mass (MM) data for polydisperse polymers by size exclusion chromatography matrix assisted laser desorptinn/ionization time-of-flight (SEC/MALDI-TOF) involves the fractionation of samples through an analytical SEC. Selected fractions are then analysed by MALDI-TOF and the mass spectra of these nearly monodisperse samples allow the determination of M-n and M-w averages. To test the reliability of the molecular mass estimates by the SEC/MALDI-TOF method, a sample of polymethylmethacrylate (PMMA), two samples of polydimethylsiloxane (PDMS), and four samples of copolyesters, all polydisperse, were analysed. The results show that the molecular mass values of PMMA fractions obtained by MALDI-TOF are coincident with those obtained using the SEC calibration plots obtained with anionic PMMA standards. In the case of the two polydimethylsiloxanes (PDMS1 and PDMS2: linear and cyclic, respectively), two slightly differing SEC calibration plots were obtained, reflecting the different structures of the polymer chains of the two samples. The SEC traces of four copolyesters were obtained in tetrahydrofuran and CHCl3. Data on MM, MM distribution solvent effects and copolymer composition are reported. © 1998 John Wiley & Sons, Ltd. [References: 20]
133) Partially Selective Methanolysis of Sebacic Units in Biodegradable Multicomponent Copolyesters
M.S.Montaudo, G.Montaudo, C.Puglisi, F.Samperi
Macromolecular Rapid Communications
19(9),
445
- 1998
The composition and molar masses of equimolar multicomponent copolyesters, obtained from 1,4-butandiol and mixtures of succinic, adipic. sebacic, and terephthalic acids were characterized by C-13 NMR, size exclusion chromatography (SEC), matrix assisted laser desorption ionization-time of flight mass spectroscopy (MALDI-TOF-MS), and viscosimetry. These copolyesters were subjected to partial methanolysis, and the composition and sequence of the oligomers obtained in the methanolysis were determined by the analysis of their fast atom bombardment (FAB-MS) mass spectra. The comparison of the composition data obtained by FAB-MS and C-13 NMR indicates that for copolymers containing sebacic units the methanolysis is a partially selective process, and that oligomers containing sebacic units accumulate in the residue. [References:19]
134) Structural Characterization of Multicomponent Copolyesters by Mass Spectrometry
M.S.Montaudo, C.Puglisi, F.Samperi, G.Montaudo
Macromolecules
31(25),
8666
- 1998
The structural characterization and composition of five random copolyesters, originating from 1,4-butanediol and mixtures of succinic, adipic, sebacic, and terephthalic acids, were obtained by analysis of their fast atom bombardment (FAB) and their matrix-assisted laser desorption ionization (MALDI) mass spectra. Multicomponent condensation copolymers may sometimes prove difficult to characterize by conventional techniques, whereas mass spectrometry is able to handle them. Once the choice between Bernoullian or Markoffian models has been made, the determination of copolymer composition, number-average sequence length, and related quantities can be achieved by applying well-defined analytical equations. The theoretical mass spectra of multicomponent copolymers are remarkably simple, and the number of peaks appearing in each copolymer mass spectrum is easily predictable. Different kinds of spectral fitting algorithms may help in the actual computations, and it has been shown that the apparent complexity of mass spectra of copolymers is due to the presence of mass series bearing different end groups. By selecting a single mass series, one obtains an experimental spectrum immediately comparable to the theoretical one. Detailed examples, together with a discussion on the reliability of results, are given to apply the computation procedures and to gain proper understanding of the concepts involved
135) Determination of sequence and composition in poly(butyleneadipate-co-butyleneterephthlate)by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
M.S.Montaudo, F.Samperi
European Journal of Mass Spectrometry
4(6),
459
- 1998
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been used here for the analysis of an unfractionated copolymer sample of poly(butyleneadipate-co-butyleneterephthalate) (PBA/PBT). This is our first report on unfractionated samples, since previous studies by MALDI-TOF MS have concerned copolymer fractions. Mass-resolved peaks are seen up to 8000 Da, The peaks are due to oligomers which have a methylester and an hydroxy as end groups (CH3O/OH), which desorb by uptake of a sodium cation. We compared the intensity of mass spectral peaks with the theoretical intensities generated using a Bernoullian distribution of sequences. The composition which gives the best match was searched by the MACO4 program and a value of 49/51 in favor of terephthalic units was found, in excellent agreement with the composition derived from the H-1 NMR data
136) Molecular Weight Determination and Structural Analysis in Polydisperse Polymers by Hyphenated Gel Permeation Chromatography/Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
International Journal of Polymer Analysis and Characterization
3,
177
- 1997
A method for the determination of molecular weight distribution data for polydisperse polymer samples by MALDI-TOF is reported that involves the fractionation of polydisperse samples through analytical GPC columns and collection of fractions. Selected fractions are then analysed by MALDI-TOF and the mass spectra of these nearly uniform samples allow the determination of M-n and M-w averages. The GPC tracing, calibrated against molecular weight values obtained by MALDI, was used to compute molecular weight distribution data of the unfractionated sample. To test the reliability of the molecular weight estimates by the GPC/MALDI-TOF method, a sample of poly(methyl methacrylate) and two samples of poly(dimethyl siloxane), both with wide polydispersity, were analyzed. The results show that the molecular weights of PMMA fractions obtained by MALDI coincide with the GPC calibration plots obtained with anionic PMMA standards, In the case of the two poly(dimethyl siloxanes), two slightly different GPC calibration plots were obtained owing to the different structures of the polymer chains of the two samples. In fact, the MALDI spectra of low MW fractions of these polymers, showed that one sample consists essentially of linear oligomers, whereas the other sample contained cyclic oligomers
137) Characterization of End Groups in Nylon 6 by MALDI-TOF Mass Spectrometry
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Journal of Polymer Science part A: Polymer Chemistry
34(3),
439
- 1996
Four samples of Ny6, each terminated by different end groups, i.e., diamino terminated, monoamino terminated (monocapped), dicarboxyl terminated, and amino-carboxyl terminated, were synthesized and analyzed by MALDI-TOF Mass Spectrometry, in order to accurately characterize their structure by direct identification of mass resolved chains. A self-calibrating method for the MALDI-TOF mass spectra of polymeric samples was used in order to distinguish the end groups existing in the four samples of Ny6. The MALDI-TOF spectra showed the presence of protonated, sodiated, and potassiated ions that were assigned to Ny6 chains containing the expecteted end groups. Furthermore, the MALDI-TOF spectra made possible the simultaneous detection of the cyclic oligomers of Ny6 present in these samples, thus achieving the full structural characterization of the molecular species present in these polyamides. © 1996 John Wiley & Sons, Inc. [References: 21]
138) Synthesis and Characterization of POLY(ETHER KETONE) POLY(ETHER SULFONE) Alternating and Sequential Copolymers by Electrophilic Reactions
P.Finocchiaro, G.Montaudo, P.Mertoli, C.Puglisi, F.Samperi
Macromolecular Chemistry and Physics
197(3),
1007
- 1996
The synthesis and NMR characterization of some poly(ether ketone)/poly(ether sulfone) (PEK/PES) alternating copolymers together with a series of sequential homopolymers containing CO, O, S and SO2, bridging groups, all obtained by electrophilic condensation reactions using phosphorus pentoxide/methanesulfonic acid as dehydrating agent, is here reported. Properties of such polymers are compared with those of their model compounds from which it can be inferred that both proton and carbon chemical shifts are very sensitive to the electronic environment generated by the O, S, CO and SO2 bridges. Differential scanning calorimetry indicates that our polymers are essentially amorphous irrespective of the bridging sequence. From viscosity measurements the molecular weights of these copolymers are judged to be very low. [References: 31]
139) Synthesis of AB and ABA Block Copolymers as Compatibilizers in Nylon 6 Polycarbonate Blends
G.Montaudo, C.Puglisi, F.Samperi, F.P.Lamantia
Journal of Polymer Science part A: Polymer Chemistry
34(7),
1283
- 1996
Nylon 6 (Ny6) and Bisphenol A polycarbonate (PC) are immiscible and form biphasic blends. To improve the compatibility of Ny6 and PC several ABA and AB Ny6/PC block copolymers were synthesized, and their compatibilizing behavior on the blends were tested. Block copolymers were prepared by reacting monoamino- or diamino-terminated Ny6 homopolymers with high molecular weight PC at 130 degrees C in anhydrous DMSO. The reaction of diamino- and monoamino-terminated Ny6 with polycarbonate produces block copolymers of the type PC-Ny6-PC (ABA) and PC-Ny6 (AB), respectively, plus a certain amount of unconverted PC degradated to lower molecular weights. To separate the block copolymer from the unconverted PC, a selective fractionation with tetrahydrofuran (THF) and trifluoroethanol (TFE) was carried out. Three different fractions were obtained: THF-soluble fraction, TFE-soluble fraction, and the TFE-insoluble fraction. The scanning electron microscopy (SEM) analysis of a 75/25 (wt/wt) Ny6/PC blend added with 2% of ABA or AB block copolymers, showed the presence of smaller PC particles more adherent to the polyamide matrix, with respect to the same blend nonadded, which is clearly biphasic. The size of the PC particles decreases from ABA to AB compatibilized blends and the adhesion with the matrix is increases in the same way. © 1996 John Wiley & Sons, Inc. [References: 26]
140) Evidence for ester exchange reactions and cyclic oligomers formation in the ring opening polymerization of lactide with Aluminium complex initiators
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi, N.Spassky, A.LeBorgne, M.Wisniewski
Macromolecules
29(10),
6461
- 1996
Four polylactide samples, obtained by ring-opening polymerization with an aluminum alkoxide initiator derived from a Schiffs base, were characterized by MALDI-TOF mass spectrometry. The MALDI mass spectra of these polylactides show well-resolved signals that can be reliably assigned to polylactide oligomers. Remarkably, both even-membered and odd-membered oligomers are present in these MALDI spectra. The presence of odd-membered oligomers cannot be explained on the basis of the lactide ring-opening polymerization, and one must admit that ester-exchange reactions do occur parallel to the polymerization process, causing a random cleavage of the polylactide chain. Furthermore, evidence for the presence of cyclic lactides was found in the MALDI-TOF spectrum of a low molecular weight polylactide fraction, indicating that ester exchange occurs also in polylactides by intramolecular end-biting reactions (ring-chain equilibration), with formation of cyclic oligomers. [References: 31]
141) End groups characterization of Poly(methylphenylsilane) by alkali metal salts doped MALDI-TOF mass spectra
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi, M.Sepulchre
Macromolecular Chemistry and Physics
197(9),
2615
- 1996
The end groups of a sample of poly(methylphenylsilane) (PMPS) obtained by the Wurtz coupling reaction of diorganodichlorosilane were characterized by MALDI-TOF mass spectrometry. Three fractions were obtained by fractionation of a PMPS sample with methanol, in order to get information on the distribution of end groups as a function of the molecular weight. The MALDI mass spectra of these fractions showed well-resolved signals up to 2500 Da corresponding to open chain oligomers of PMPS bearing four different types of end groups: -Si-(CH3)(3), -O-Si-(CH3)(3), -O-CH3, -H. Six different types of linear oligomers were identified together with a sizeable amount of cyclic oligomers, most likely generated during the polymerization step. The problem of isobar mass series in the MALDI spectra due to clusters of oligomers with Li+, Na+, K+ was solved by comparing the MALDI spectra of PMPS fractions with and without the addition of a specific alkaline salt (KCl or NaI) to the solution containing the sample and matrix. The intensity of the oligomer clusters with the specific cation were enhanced with respect to the others. The mass shifts observed in the MALDI-TOF doped spectra with respect to the undoped ones allowed the unambiguous assignments of the isobar peaks corresponding to PMPS oligomers. [References: 22]
142) Sequence Distribution of PES/PEK Copolymers by MS and by 13C-NMR.
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Macromolecular Chemistry and Physics
196(2),
499
- 1995
The sequence distributions of four poly(ether-sulfone)/poly(ether-ketone) (PES/PEK) copolymer samples were determined by means of fast atom bombardment mass spectrometry (FAR-MS) and by C-13 NMR spectroscopy. The controlled partial degradation of the copolymer chains to produce oligomers suitable of FAB-MS analysis was performed with sodium methoxide in dimethyl sulfoxide solution at 130 degrees C, and the experimental conditions were optimized. The sequential arrangements of ether-sulfone/ether-ketone units present in these materials were estimated by a best-fit minimization method using the MACO4 computer program which compares the experimental FAB-MS spectral intensities with theoretical intensities. Random PES/PEK copolymer samples showed quite the same sequence arrangements as expected from monomer feed-ratios used in the syntheses. Instead, a PES/PEK copolymer sample expected to be exactly alternating (from the synthesis procedure) showed 44% of random sequences owing to the transetherification reaction which occurred in the synthesis. The results of sequential analysis obtained from C-13 NMR data compare well with the data obtained by the FAB-MS analysis. [References: 29]
143) Characterization of Polymers by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry-End Group Determination and Molecular Weight Estimates in Poly(ethylene glycols)
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Macromolecules
28,
4562
- 1995
"Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) allows mass measurements of large molecules such as those present in synthetic and natural macromolecules. We have used a self-calibrating method for the MALDI-TOF spectra of polymers, enabling us to obtain: accurate mass values. Poly(ethylene glycol) (PEG) samples having two different kinds of end groups were used in our work: (i) anionic PEG, H-(CH2-CH2-O)(n)-H; (ii) cationic PEG, H-(CH2-CH2-O)(n)-OH. The MALDI-TOF spectra recorded in reflected mode allowed unambiguous identification of the end groups present in these PEG samples. Five anionic PEG samples with very narrow molecular weight distributions (MWD) were studied. The MALDI-TOF spectra of PEGs were recorded in both linear and reflected modes. Due to the high sensitivity and the highly linear response of HIMAS (a microchannel detector equipped with a photomultiplier), measurements of MALDI-TOF spectra were used in our work. To estimate the MW and MWD of the PEG samples. Our results show that the molecular weight estimates provided by MALDI-TOF measurements agree with the values obtained by conventional techniques such as GPC, osmometry, and viscometry. [References: 23]"
144) Characterization of Polymers by Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry: Molecular Weight Estimates in Samples of Varying Polydispersity
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
9(5),
453
- 1995
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) allows the detection of large molecules such as those present in synthetic and natural macromolecules. Synthetic polymers may show a wide range of molecular weight distributions (MWD), according to the synthetic method used in their preparation. We have studied several poly(methylmethacrylate) (PMMA), poly(styrene) (PS) and poly(ethyleneglycol) (PEG) samples with varying MWD, and also a number of condensation polymers such as Nylon-6, poly(carbonate) and poly(ester). Measurements of MALDI-TOF spectra in the linear mode were used in our work to estimate the molecular weight (MW) and MWD of the polymer samples. Our results show that the molecular weight estimates provided by MALDI-TOF measurements agree with the values obtained by conventional techniques, such as gel-permeation chromatography (GPC), only in the case of polymer samples with very narrow molecular weight distribution. However, when the polydispersity reaches values around 1.10 the difference between the MW measured by GPC and measured by MALDI may amount to up to about 20%. At higher dispersions, the MALDI spectra fail to yield reliable MW values. [References: 29]
145) Molecular Weight Distribution of Poly(dimethylsiloxane) by Combining Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry with Gel-permeation Chromatography Fractionation
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
9(12),
1158
- 1995
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) allows detection of large molecules such as those present in synthetic and natural macromolecules. Until recently, it was reported that MALDI-TOF measurements can provide correct molecular weight (MW) estimates only for nearly monodisperse polymer samples. We have now developed a methodology for polydisperse samples. We recorded the gel-permeation chromatography (GPC) trace of a polydisperse polymeric sample of poly(dimethylsiloxane) (PDMS), collecting 42 fractions. Selected fractions were analyzed by MALDI-TOF and the average MW of each fraction was determined, allowing a calibration of GPC curves against absolute MW. The calibrated GPC trace was then used to compute average MW and molecular-weight distribution (MWD) of the unfractionated poly(dimethylsiloxane) sample. In the spectra of low molecular-weight fractions, the resolution is high enough to resolve the contributions of the various PDMS oligomers as separate signals. [References: 26]
146) Molecular and Structural Characterization of Polydisperse Polymers and Copolymers by Combining Maldi-Tof Mass Spectrometry with GPC Fractionation
G.Montaudo, D.Garozzo, M.S.Montaudo, C.Puglisi, F.Samperi
Macromolecules
28,
7983
- 1995
Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) allows detection of large molecules such as those present in synthetic and natural macromolecules. Until recently, it was reported that MALDI-TOF measurements can provide correct molecular weight (MW) averages only for samples with a narrow MW distribution (M(w)/M(n) less than or equal to 1.20). We have now developed a methodology for polydisperse samples. We recorded the GPC trace of two polydisperse polymeric samples, namely, poly(butylene adipate) (PEA) and poly(butylene adipate-co-butylene succinate) (PBAS), collecting about 40-50 fractions per run. Selected fractions were analyzed by MALDI-TOF, and the average MW of each fraction was determined, allowing calibration of the GPC curves against absolute MW. The two calibrated GPC traces were then used to compute average MW and molecular weight distributions (MWD) of the unfractionated samples. End group analysis from MALDI-TOF spectra revealed that the PEA sample is composed of seven different types of chains. For the copolymer sample, PBAS, analysis of the MALDI spectra established a random sequence distribution of comonomer units. The succinate/adipate molar ratio calculated from the MALDI spectra is in good agreement with the molar ratio found by NMR. [References: 25]
147) Exchange Reactions Occurring through Active Chain Ends. Melt Mixing of Nylon 6 and Polycarbonate
G.Montaudo, C.Puglisi, F.Samperi
Journal of Polymer Science part A: Polymer Chemistry
32,
15
- 1994
The chemical reactions occurring in the melt mixing of nylon6/polycarbonate (Ny6/PC) at 240-degrees-C were investigated. The reaction of equimolar Ny6/PC blends can be reconciled within the overall scheme of an exchange reaction occurring with the attack of active amino terminals on the inner carbonate groups. We have performed the synthesis of low molecular weight amino-terminated nylon 6 and the effect of the active amino terminal groups on the exchange kinetics was investigated. The exchange reaction yields sizeable amounts of copolymer, in fact after 75 min of melt mixing the (initially equimolar) blend contains 30 mol of unreacted PC and 70 mol of Ny6/PC copolymer (all the Ny6 was therefore incorporated in the copolymer). Trifluoroacetylation of nylon 6 was used to produce CHCl3-soluble Ny6/PC copolymers, that could be analyzed by NMR. The NMR analysis yielded, beside the copolymer composition, evidence of the presence of urethane units interconnecting the Ny6 and PC blocks. The amount of urethane units increased with the reaction time, indicating a reduction of the block size as a function of the extent of exchange. Our study established the structure of the products formed, provided the materials balance of the process, and investigated some salient kinetic aspects. A thermal degradation study was also performed by thermogravimetry and direct pyrolysis mass spectrometry, to identify the products formed in the thermal treatment of the blends and to investigate the possible role of the inner amide groups in the intermolecular exchange reactions occurring between Ny6 and PC. Our results prove that these reactions occur above 300-degrees-C, and that only the cleavage of carbonate groups, by means of Ny6 amino end groups, is actually occurring at 240-degrees-C. © 1994 John Wiley & Sons Inc. [References: 31]
148) Primary Thermal Degradation Processes of PES and PPO Investigated by Direct Pyrolysis Mass-Spectrometry
G.Montaudo, C.Puglisi, R.Rapisardi, F.Samperi
Macromolecular Chemistry and Physics
195,
1225
- 1994
The thermal degradation processes which occur in poly(ether-sulfone) (PES) and poly(phenylene oxide) (PPO) have been studied by Direct Pyrolysis-Mass Spectrometry (DPMS). The pyrolysis mass spectra of PES and PPO contain molecular ions up to m/z 700, corresponding to pyrolysis compounds of sufficient size to allow the characterization of the primary processes which occur in the thermal cleavage of these polymers. The results obtained indicate that the thermal degradation of PES and PPO proceeds by thermal cleavage of the groups located at the bridges. In the case of PPO the bridges are all homogeneous, and therefore both an intramolecular thermal rearrangement or a radical cleavage process can account for the formation of the primary pyrolysis products. Formation of dibenzofuran units along the PPO chain is a parallel thermal process. In the case PES the simultaneous thermal cleavage of two different bridged units, diphenyl sulfone and diphenyl ether, occurs. Extrusion of SO2, with consequent formation of compounds containing biphenyl units, is observed in the thermal degradation of PES. This process is accompanied by formation of dibenzofuran units along the polymer chain, as expected from the thermal cleavage of diphenyl ether units, and by another rearrangement of the polymer backbone, yielding structures containing more oxygen atoms with respect to that of PES. [References:23]
149) Primary Thermal Degradation Processes of PEK and PEK/PES Copolymers Investigated by Direct Pyrolysis Mass-Spectrometry
G.Montaudo, C.Puglisi, F.Samperi
Macromolecular Chemistry and Physics
195,
1241
- 1994
The mechanisms of thermal degradation of poly(ether-ketone) (PEK) and four poly(ether-ketone)/poly(ether-sulfone) copolymers (PEK/PES) have been investigated by direct pyrolysis-mass spectrometry (DPMS). Several families of pyrolysis compounds with H, OH and CHO end-groups have been identified in the pyrolysis mass spectra of PEK. All these pyrolysis compounds can arise from degradation mechanisms involving cleavages of the bridged groups (diphenyl ether and dibenzophenone units). Our data show that the main degradation products of PEK are aldehydes, most likely formed by an intramolecular thermal cleavage of benzophenone units. Compounds containing dibenzofuran units have also been observed in the DCI mass spectrum of PEK. The thermal decomposition of a low molecular weight PEK sample occurs in two stages with the maxima of decomposition at 390-degrees-C and 490-degrees-C, respectively. This fact indicates the occurrence of an end-group initiated thermal decomposition in the early degradation stage which is not present in the case of the high molecular weight PEK sample. The pyrolysis of PEK does not produce compounds containing biphenyl units, indicating that extrusion of carbonyls or recominbation processes are not involved. The thermal degradation compounds of the PEK/PES copolymers originate from the thermal cleavage of the bridge groups (diphenyl ether, benzophenone and diphenyl sulfone). The pyrolysis mass spectra of 1 : 1 (alt.), 1 : 1 (random), 3 : 1 and 1 : 3 PEK/PES copolymers appear essentially identical (apart for obvious differences in peak intensities), indicating that the molecular rearrangements (SO2 extrusion, transesterification, cleavage of bridges) which occur at higher temperatures and/or in the pyrolysis processes are able to randomize the distribution of comonomer units originally present in the copolymers. Differences in peak intensities have been found to reflect almost quantitatively the molar composition of the copolymers. [References: 16]
150) Primary Thermal Degradation Processes Occurring in Polyphenylenesulfide Investigated by Direct Pyrolysis Mass-Spectrometry
G.Montaudo, C.Puglisi, F.Samperi
Journal of Polymer Science part A: Polymer Chemistry
32,
1807
- 1994
"The thermal degradation processes which occur in poly(phenylenesulfide) (PPS) have been studied by direct pyrolysis-mass spectrometry (DPMS). The structure of the compounds evolved in the overall temperature range of PPS decomposition (400-700-degrees-C) suggests the occurrence of several thermal decomposition steps. At the onset of the thermal degradation (430-450-degrees-C) this polymer decomposes with the formation of cyclic oligomers, generated by a simple cylization mechanism either initiated at the - SH end groups or by the exchange between the inner sulfur atoms along the polymer chain. At higher temperature (>500-degrees-C) another decomposition reaction takes over with the formation of aromatic linear thiols. The formation of thiodibenzofuran units by a subsequent dehydrogenation reaction occurs in the temperature range of 550-650-degrees-C; in fact, pyrolysis products with a quasi-ladder structure have also been detected. Ultimately, above 600-degrees-C, extrusion of sulfur from the pyrolysis residue occurs with the maximum evolution at the end of decomposition (about 700-degrees-C). It appears, therefore, that the residue obtained at high temperature tends to have a crosslinked, graphite-like structure from which the bonded sulfur is extruded. © 1994 John Wiley & Sons, Inc. [References: 18]"
151) Synthesis and NMR Characterization of a Series of Linear Monodisperse Poly(Aryl Ether Ketone) and Poly(Aryl Ether Sulfone) Oligomers
P.Finocchiaro, G.Montaudo, P.Mertoli, F.Samperi
Macromolecular Chemistry and Physics
195(8),
2779
- 1994
New poly(aryl ether ketone) and poly(aryl ether sulfone) monodisperse model oligomers were synthesized in good yield via nucleophilic reactions. They were characterized by means of H-1 and C-13 NMR spectroscopy, and all chemical shifts were assigned to the corresponding nuclei. Both proton and carbon chemical shifts are very sensitive to the electronic environment generated by the O, CO and SO2 bridges, but in all oligomers, nuclei with similar electronic environments are very close to each other no matter what the sequence or the length of the oligomer is. Only quaternary carbons are more sensitive to the nature of the bridging groups, and therefore their chemical shifts can be of utility in sequencing the polymers. [References: 16]
152) Poly(Arylenecarbonate)s Oligomers by Carbonate Interchange Reaction of Dimethyl Carbonate with Bisphenol-A - FAB-MS Evidence for the Nature of End Groups in the Oligomers
A.G.Shaikh, S.Sivaram, C.Puglisi, F.Samperi, G.Montaudo
Polymer Bulletin
32(4),
427
- 1994
Fast atom bombardment mass spectrometry (FAB-MS) has been used to identify oligomers with different end groups obtained by the carbonate interchange reaction of bisphenol-A with dimethyl carbonate and the partial methanolysis of poly(bisphenol-A carbonate)s. Based on the FAB-MS data, a reaction pathway for the formation of compounds in the synthesized oligomers is proposed.
153) Determination of Absolute Mass Values in MALDI-TOF of Polymeric Materials by a Method of Self-Calibration of the Spectra
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Analytical Chemistry
66(23),
4366
- 1994
A novel calibration procedure is introduced that allows one to obtain absolute mass values in MALDI-TOF spectra of polymeric materials. The masses of the oligomers are written as the sum of two masses: mu(n) = sigma(n) + theta, where theta is the mass of the first oligomer and sigma(n) is a multiple of the polymer repeat unit and represents the distance in daltons from the first oligomer. The new calibration procedure considers theta as a variable parameter and performs a best-fit minimization. Two examples of application of the new procedure are given. Two synthetic polymers, poly(caprolactone) and poly(Bisphenol A carbonate), were analyzed by MALDI-TOF, and complex spectra showing one or more mass series up to 20 kDa were obtained. Application of this procedure allowed the interpretation of each single peak in the mass spectra and established that none of the peaks in the spectrum corresponds to fragments. It was found that the poly(caprolactone) sample consists of a series of oligomers with H and OH as terminal groups, which form sodium-cationized ions in the MALDI process. The composition of the poly(Bisphenol A carbonate) sample was more complex, since it contains a series of cyclic oligomers desorbing as sodium-cationized ions, plus two series of oligomers bearing H/phenol and phenyl/phenol as terminal groups, respectively, which form ions by capturing a proton in the MALDI process. [References: 11]
154) Self Calibrating Property of Matrix-assisted Laser Desorption/Ionization Time-of-flight Spectra of Polymeric Materials
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
8(12),
981
- 1994
A calibration procedure is introduced, which allows one to obtain absolute mass values in matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) spectra of polymeric materials, thus avoiding ambiguous mass assignments, The method consists in combining the observed times of flight in a function denoted as 'modified sum of squares' (SS2) and in finding the point of minimum for SS2. The procedure takes advantage of the finding that SS2 possesses an extremum property, i.e. it takes its minimum at theta, the mass of the first oligomer appearing in the MALDI-TOF spectrum, The method was tested by recording the MALDI-TOF spectrum of an aminolyzed Nylon 6 and of a glycolized poly(butyleneadipate) (PEA) sample. Nylon 6 was found to be made of a series of oligomers bearing diaminohexamethylene (DAHM) terminal groups. PEA is made of a series of oligomers with butyleneglycol end-groups. The intense peaks seen in the mass spectra are due to sodium-cationized ions, whereas the less-intense peaks are due to potassium-cationized ions. The function SS2 was built for the two samples and a point of minimum was found at the point theta=817.6 for Nylon 6 and at the point theta=713,6 for PBA. This finding allowed the interpretation of the mass spectra. [References: 15]
155) 2-(4-Hydroxyphenylazo)-benzoic acid : A Solid Matrix for Matrix-assisted Laser Desorption/Ionization of Polystyrene
G.Montaudo, M.S.Montaudo, C.Puglisi, F.Samperi
Rapid Communications in Mass Spectrometry
8(12),
1011
- 1994
Liquid matrices are currently used in matrix assisted laser desorption ionization (MALDI) of non-polar macromolecules, such as polystyrene. We investigated the possibility of using non-liquid compounds as matrices. A series of polystyrene samples was analyzed by MALDI using a time-of-flight (TOF) analyzer. We found that a solid matrix, namely 2-(4-hydroxyphenylazo)-benzoic acid, yields quality mass spectra. The MALDI-TOF spectra were used to measure the parameters which characterize the molecular weight distributions of the polystyrene samples. The values obtained by MALDI-TOF were in good agreement with those obtained by other methods. [References: 12]
156) Chemical Reactions Occurring the Thermal Treatment of Polymer Blends Investigated by Direct Pyrolysis Mass Spectrometry. Polycarbonate/Polybuthyleneterefthalate
G.Montaudo, C.Puglisi, F.Samperi
Journal of Polymer Science part A: Polymer Chemistry
31,
13
- 1993
The chemical reactions occurring in the thermal treatment of polycarbonate/polybuthyleneterephthalate (PC/PBT) blends have been investigated by gradual heating (10-degrees-C/min) using thermogravimetry and direct pyrolysis into the mass spectrometer. Exchange reactions occur already in the temperature range below 300-degrees-C but the transesterification equilibrium is affected by the evolution of thermal degradation products. Buthylenecarbonate, was detected in the first decomposition stage (320-380-degrees-C), which is evolved together with a series of cyclic compounds containing units of PC and PBT, in varying ratios. The overall thermal reaction evolves towards the formation of the most thermally stable polymer, i.e., a totally aromatic polyester (polymer III, Table I), which was found to be the end-product of the thermal processes occurring in the system investigated. The thermal decomposition products obtained from the PC/PBT blends in the range 320-600-degrees-C have mass sufficiently high to be structurally significant, since they contain at least one copolymer repeating unit. The reactions occurring in the thermal treatment of the PC/PBT blend are discussed in detail
157) Primary Thermal Degradation Mechanisms of PET and PBT
G.Montaudo, C.Puglisi, F.Samperi
Polymer Degradation and Stability
42(1),
13
- 1993
The primary thermal degradation mechanisms of polyethyleneterephthalate (PET) and polybutyleneterephthalate (PBT) have been studied by direct pyrolysis mass spectrometry using negative chemical ionization (NCI). The NCI mass spectra of PET and PBT are dominated by series of ions which might be assigned either to cyclic or open-chain oligomers with olefin and carboxylic end-groups. The structures of the pyrolysis products have been identified by comparison of their daughter ion spectra with those of authentic samples of cyclic and open-chain oligomers with olefin and carboxyl end-groups. The authors' results provide strong evidence that cyclic oligomers are the primary pyrolysis products of PET and PBT, formed by intramolecular exchange (ionic) reactions. These further decompose by a beta-CH hydrogen transfer reaction, which involves a six-membered cyclic transition state, and generates open chain oligomers with olefin and carboxylic end-groups. The pyrolysis of PET and PBT was also carried out in the presence of ammonium polyphosphate (APP) (an acid precursor). APP affects the pyrolysis by increasing the rate of decomposition and lowering the Polymer Decomposition Temperatures (PDT)s of both polymers. However, it does not change the nature of the compounds obtained in the pyrolysis of the pure polymers. This fact provides a firm argument in favour of the belief that the beta-CH hydrogen transfer occurs by an ionic process. [References: 60]
158) Further-Studies on the Thermal-Decomposition Processes in Polycarbonates
G.Montaudo, C.Puglisi, R.Rapisardi, F.Samperi
Polymer Degradation and Stability
31(2),
229
- 1991
The thermal decomposition mechanisms of poly(trimethylene carbonate), poly(neopentylene carbonate), poly(2-phenyltrimethylene carbonate) and poly(p-xylylene carbonate) have been investigated by EI/CI direct pyrolysis mass spectrometry and by desorption chemical ionization (DCI) mass spectrometry. The results indicate that all four polycarbonates decompose by an intramolecular exchange, an ionic process that produces cyclic carbonates as pyrolysis products. Other thermal processes (Reactions 1c-1i, Scheme 1) also occur during the pyrolysis, and are all explained on the basis of well-established ionic reactions (Scheme 1)
159) Chemical-Reactions Which Occur in the Thermal-Treatment of Polycarbonate Polyethyleneterephthalate Blends, Investigated by Direct Pyrolysis Mass-Spectrometry
G.Montaudo, C.Puglisi, F.Samperi
Polymer Degradation and Stability
31(3),
291
- 1991
The chemical reactions which occur in the thermal treatment of polycarbonate/poly(ethyleneterephthalate) (PC/PET) blends have been investigated by gradual heating (10-degrees-C/min) using thermogravimetry and direct pyrolysis into the mass spectrometer. Exchange reactions occur even in the temperature range below 300-degrees-C, but the transesterification equilibrium is affected by the evolution of thermal degradation products. Ethylene carbonate was detected in the first decomposition stage (280-380-degrees-C), and is evolved together with a series of cyclic compounds containing units of PC and PET in varying ratios. The overall thermal reaction evolves towards the formation of the most thermally stable polymer, i.e. a totally aromatic polyester (polymer III, Table 1), which was found to be the end product of the thermal processes occurring in the system investigated. Several thermal decomposition products, originating from the PC/PET blends in the range 250-600-degrees-C, were identified. The compounds detected have masses sufficiently high to be structurally significant, since they contain at least one copolymer repeating unit. The reactions which occur during the thermal treatment of the PC/PET blend are discussed in detail
160) Sequencing an aromatic copolycarbonate by partial ammonolysis and FAB-MS analysis of the products
G.Montaudo, C.Puglisi, F.Samperi
Polymer Bulletin
21(5),
483
- 1989
Fast-atom bombardment mass spectrometry (FAB-MS) was used to identify the low-mol.-wt. compds. produced in the partial piperidine aminolysis of an essentially alternating copolycarbonate contg. resorcinol and bisphenol A (I) units. The FAB-MS spectra allowed identification of sequences with an exactly alternating structure. Some spurious sequences contg. blocks of 2-4 consecutive I units were also detected.
161) Thermal decomposition processes in polycarbonates
G.Montaudo, C.Puglisi, F.Samperi
Polymer Degradation and Stability
26(3),
285
- 1989
The thermal decompn. mechanisms of some polycarbonates were studied by direct pyrolysis mass spectrometry. Aliph. polycarbonates decompd. by intramol. exchange processes which produce cyclic compds. as primary pyrolysis products. Aliph.-arom. polycarbonates yielded cyclic compds. contg. various ratios of bisphenol A (I) and aliph. carbonates. Bisphenol A bischloroformate-resorcinol copolymer underwent ester-exchange rearrangement to produce cyclic resorcinol carbonate oligomers and I polycarbonate.
 Top
Top
















 After a decade from the discovery of poly-BF1, the present chapter narrates the history of polybenzofulvene derivatives from the discovery to the latest developments. Now that more than 50 polymers belonging to this family have been synthesized and the relevant studies have been published in the most important journals dealing with polymer science, the spontaneous polymerization of benzofulvene monomers and the special features of the corresponding polymers appear to become a well-established topic. The chapter provides an in-depth analysis of the literature on the subject, from the preparation methods to the characterization of polybenzofulvene derivatives, the study of their properties, and the evaluation of the possible applications.
After a decade from the discovery of poly-BF1, the present chapter narrates the history of polybenzofulvene derivatives from the discovery to the latest developments. Now that more than 50 polymers belonging to this family have been synthesized and the relevant studies have been published in the most important journals dealing with polymer science, the spontaneous polymerization of benzofulvene monomers and the special features of the corresponding polymers appear to become a well-established topic. The chapter provides an in-depth analysis of the literature on the subject, from the preparation methods to the characterization of polybenzofulvene derivatives, the study of their properties, and the evaluation of the possible applications.


 The application of different polymerization procedure of hte commercial aromatic bisphenol-A polycarbonate (referred herein as PC) and the innovative enzymatic catalysed polymerization of aliphatic polycarbonate are summarized. Due to the high engineering performance of PC polymer, an extensive section on mechanical, electrical, chemical and therma properties is included. The thermo and photo oxidative behaviours, the hydrolytic stability and the consequent modification on PC chemical structure are also discussed. The development of PC polymeric materials such as composites and blends are also addressed, emphasizing in particular the properties and the applications of impact modified PC blends and even of PC/Polyester systems.
The application of different polymerization procedure of hte commercial aromatic bisphenol-A polycarbonate (referred herein as PC) and the innovative enzymatic catalysed polymerization of aliphatic polycarbonate are summarized. Due to the high engineering performance of PC polymer, an extensive section on mechanical, electrical, chemical and therma properties is included. The thermo and photo oxidative behaviours, the hydrolytic stability and the consequent modification on PC chemical structure are also discussed. The development of PC polymeric materials such as composites and blends are also addressed, emphasizing in particular the properties and the applications of impact modified PC blends and even of PC/Polyester systems.
